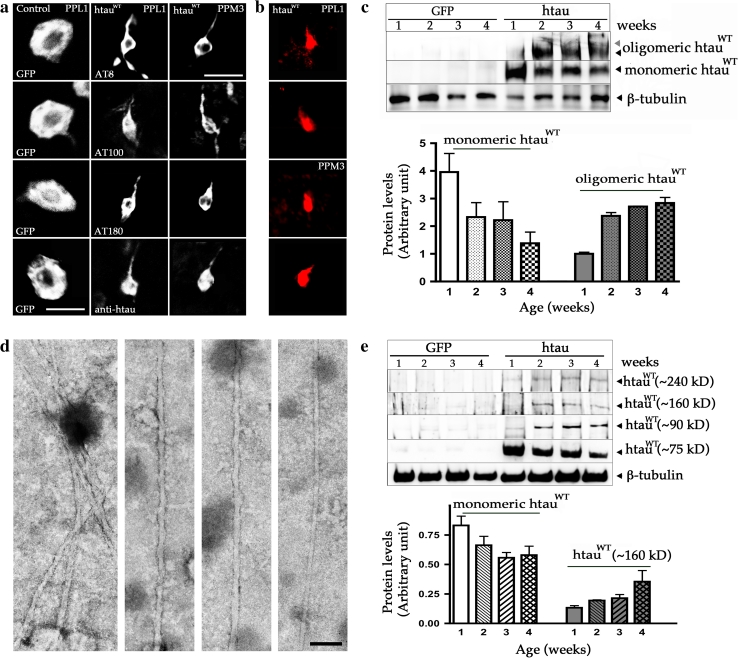
Fig. 4.
Expression of htauWT produces AD-like abnormal tau phosphorylation and NFT-like pathology. a Single cell of PPL1 and PPM3 DA neurons from fly brains at 6 weeks of age. Compared to the age-matched control DA neurons from PPL1 cluster labeled with GFP (left column), immunostaining of three antibodies that recognize AD-like hyperphosphorylated tau (AT8 top row, AT100 top second row, AT180 bottom second row), and a polyclonal tau antibody (bottom row) reveal tangle-like pathology in the degenerating DA neurons from PPL1 (second column) and PPM3 (third column) clusters. b The degenerating DA neurons from both PPL1 and PPM3 clusters are stained with Congo red. The time course of pathological tangle-like structure formation in htauWT brains during the aging process is presented in Fig. S3. c Representative immunoblot of polyclonal anti-htau shows that a substantial amount of htauWT proteins are converted from monomer tau (75 kDa) to the oligomeric Tau species (>400 kDa). Anti-β-tubulin serves as a loading control. Protein expression levels from four independent immunoblots with monomeric and oligomeric htau protein levels calibrated against the loading control are shown as relative expression. Values shown represent mean ± SEM. d Electron micrographs of abnormal filaments extracted from tangle-rich preparation (sarkosyl-insoluble pellets) of fly brains expressing htauWT. Images show several PHF-like filaments are bundled (left panel), a single PHF (middle panels), and a straight filament (right panel). Scale bar 100 nm. d Representative immunoblot from sarkosyl-insoluble pellets reveals monomer tau ***(75 kD) and the htau-containing species with molecular weights range between 75 and 400 kD (~90, ~160 and ~240 kD). Anti-β-tubulin also serves as a loading control. e Monomeric and polymeric htau protein levels are quantified by analyzing three independent immunoblots and shown as relative expression. Values represent mean ± SEM