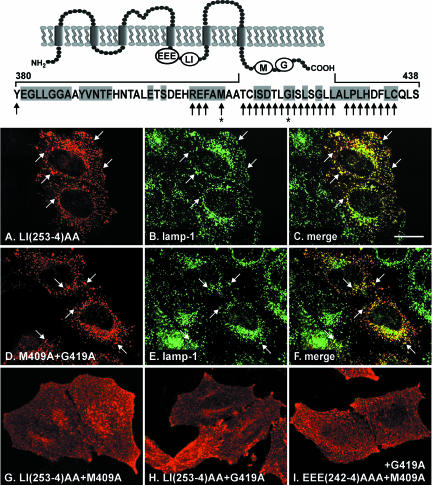
Figure 5.
The lysosomal targeting motifs of CLN3 protein. Full-length CLN3 proteins carrying different alanine substitutions were transiently expressed in HeLa cells, and localization of the mutated proteins was investigated by double labeling with CLN3 specific antibody (33aff) and antibody to lamp-1 (H4A3) followed by confocal microscopy. CLN3 carrying the LI(253-4)AA substitution alone (A) was mostly targeted to lysosomes (A), colocalizing well with lamp-1 (B); merged images shown in C. Single alanine scanning mutagenesis was carried out along the tail domain of CLN3 in the presence or absence of the LI(253-4)AA substitution in the loop domain (mutated amino acids are indicated by black arrows along the sequence given above confocal images). CLN3 carrying both M409A+G419A alone (intact LI) was localized to lysosomes (D, CLN3; E, lgp120; and F, merged images). When expressed in conjunction with the LI(253-4)AA mutation the two separate amino acid substitutions (asterisks), M409A (G) and G419A (H), resulted in mistargeting of CLN3 to the plasma membrane. When M409A and G419A mutations were both present together with intact LI(253-4), additional alanine substitutions in the acidic patch EEE (242-4)AAA upstream of the LI-motif resulted in a protein that was again mostly mistargeted to plasma membrane (I). Examples of lysosomal colocalization of CLN3 and lgp120 are indicated by white arrows in A-F. Bar, 10 μM. Topology diagram: positions of important amino acid motifs, EEE(242-4), LI(253-4), M409, and G419 are indicated. The amino acid sequence shows the most C-terminal 59 amino acids of CLN3, highly conserved residues are indicated by gray boxes.