Figure 4.
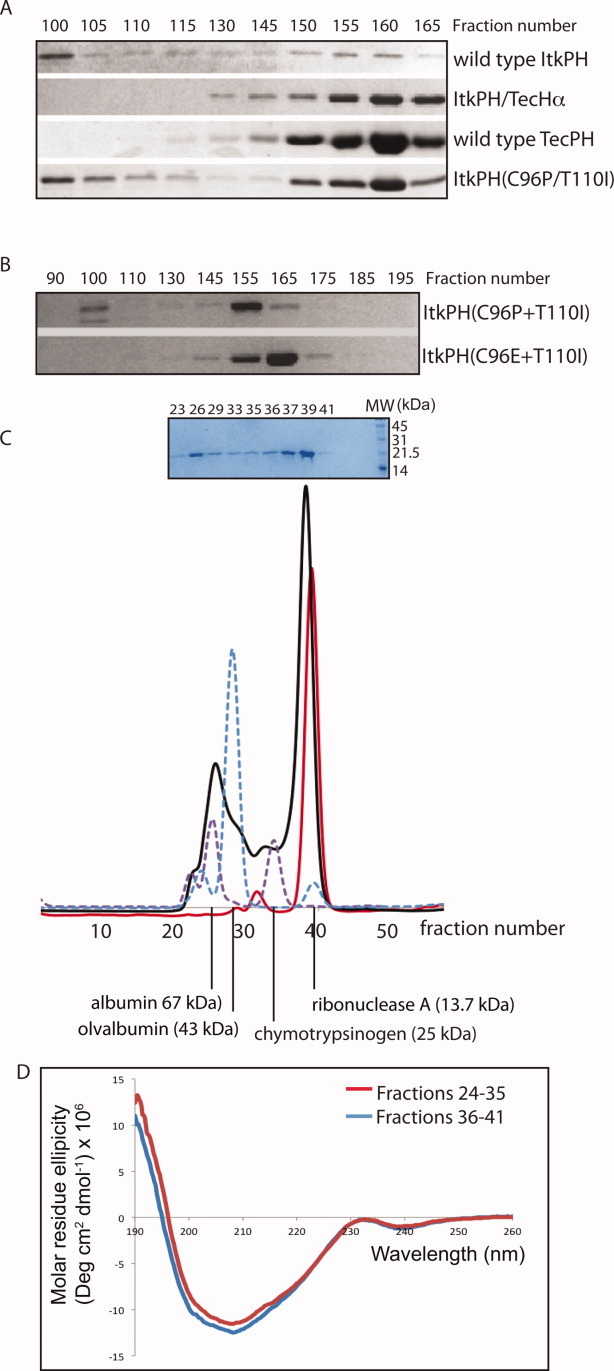
Characterization of purified PH domain proteins. (A) Four different His6-GB1 fusion proteins were purified and run through a Sephadex S300 sizing column; wild type ItkPH and ItkPH(C96P/T110I) show signs of aggregation, eluting in two peaks, whereas ItkPH/TecHα and TecPH WT each elute as a single peak corresponding to the size of the monomeric PH domain. (B) Purified His6-GB1-ItkPH(C96P/T110I) and His6-GB1-ItkPH(C96E/T110I) were run through a Sephadex S100 sizing column. Unlike ItkPH(C96P/T110I), the ItkPH(C96E/T110I) mutant elutes as a single peak. (C) After cleavage of the His6-GB1 tag, purified 13C/15N-labeled ItkPH(C96E/T110I) was passed over a Superdex-75 column on an Aktä FPLC system (solid black line). SDS-PAGE analysis of the resulting fractions is shown above the FPLC trace. Protein standards (dashed purple and blue lines) are shown and indicate the major peak for ItkPH(C96E/T110I) elutes in a manner consistent with its 19 kDa monomeric molecular weight. After completion of NMR data acquisition, the IP4-bound ItkPH(C96E/T110I) was re-injected onto the Superdex-75 column and run under identical conditions (solid red line). The slight shift in the elution profile of IP4 bound ItkPH (compare red with black trace) is consistent with previously reported findings that PH domains exhibit restricted motions on binding to IP4.31 (D) The two peaks (fractions 24–35 and 36–41) from the initial ItkPH(C96E/T110I) Superdex-75 run (black trace in (C)) were analyzed by CD spectroscopy. The two samples show no significant difference in secondary structure.
