Figure 2.
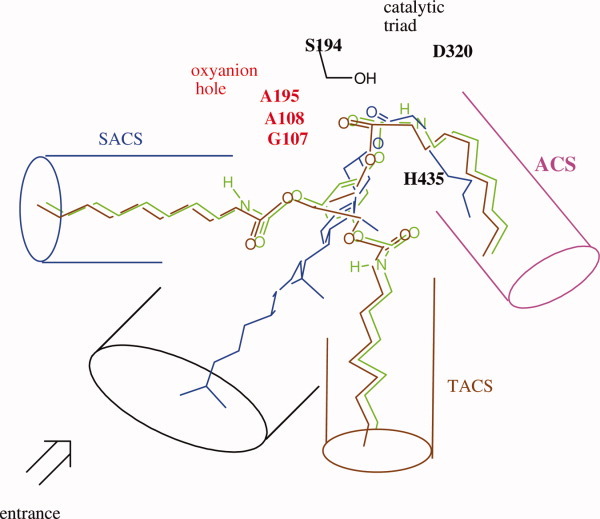
Superimposition of tridentate inhibitor 1,3,5-tri-N-n-octylcarbamyl-phloroglucinol (1), cholesterol ester, and TG into the active sites of CEase that contains the catalytic triad, an oxyanion hole, ACS, SACS, TACS, and LGS based on the X-ray crystal structures.5, 6 One of octylcarbamate moiety of inhibitor 1, the acyl chain of cholesterol ester, and the sn-1 or sn-3 acyl chain of TG are orientated to fit into ACS of the enzyme. The carbonyl oxygen atoms of these ACS-bound acyl or carbamyl groups of substrates or inhibitor are orientated to fit into the oxyanion hole of the enzyme, and the carbonyl carbon atoms of those are in the correct position for the attack by the Ser 194 of the enzyme. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
