Fig. 1. Exchange of the Ste7 MAPKK from the Ste5 scaffold protein.
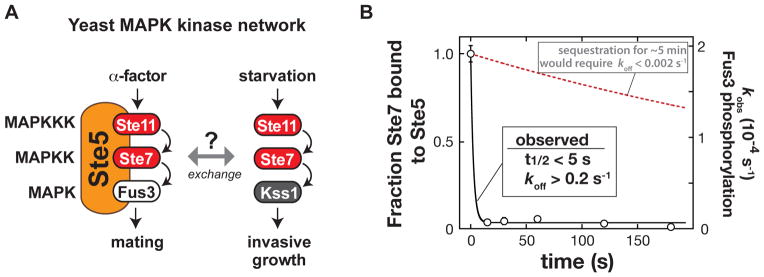
(A) Shared components of the yeast mating and invasive growth pathways yield physiologically distinct input-output responses.
(B) Dissociation rate of the MAPKK Ste7 from the Ste5 scaffold protein measured with purified recombinant Ste5, the MAPKKK Ste11, the MAPK Fus3, and a constitutively active form of the MAPKK Ste7 (Ste7EE, bearing phosphomimic mutations in the Ste7 activation loop (16)). To a preassembled Ste5-Ste11-Ste7-Fus3 complex, an excess of a Ste7 binding domain (a minimal Ste7 binding domain from Ste5 [residues 759–810]) was added to capture Ste7 as it dissociated from Ste5 (Fig. S1). At various times, ATP was added, and the initial rate of Fus3 phosphorylation was measured (the amount of Ste5-Ste7-Fus3 complex remaining at each timepoint). Error bars are standard deviations. The observed koff of 0.2 s−1 is a lower limit – dissociation occurred on a timescale faster than could be measured with mixing by hand.
