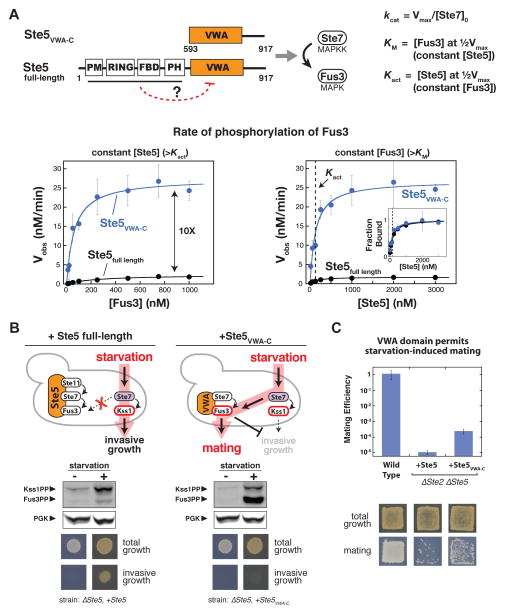
Fig. 2. Autoinhibition of the Ste5 scaffold protein insulates the MAPK Fus3 from activation by incorrect inputs.
(A) Full-length Ste5 (residues 1–917) and Ste5VWA-C (residues 593–917) used for in vitro kinetic assays for phosphorylation of Fus3 by Ste7EE, Michaelis-Menten plot of Vobs vs. [Fus3], and plot of Vobs vs. [Ste5]. Kact corresponds to the midpoint of the Vobs vs. [Ste5] plot and represents the dissociation constant for Ste5-Ste7. Error bars are standard deviations. It was unnecessary to measure binding affinity for Fus3 assembly into the ternary complex because the Ste5 VWA domain does not bind with any detectable affinity to Fus3 (Fus3 is recruited to this ternary catalytic complex via binding to Ste7; the interaction between the Ste5 VWA domain and Fus3 is a transient catalytic interaction (16)). See fig. S2-S4 and table S1 for values of fitted kinetic constants.
(B) Fus3 misactivation in response to starvation in yeast cells expressing Ste5VWA-C (see Methods for growth conditions). Fus3 and Kss1 phosphorylation was monitored with an antibody to phosphorylated MAPKs by protein immunoblotting, and phosphoglycerate kinase (PGK) is shown as a loading control for yeast lysates (see fig. S5-S7 for quantitative analysis). The invasive growth response was assayed with yeast cells grown on solid agar plates (see Methods). Similar results were obtained with constitutively active alleles of Ste11 and Ste7 (Fig. S8).
(C) Ste5VWA-C misdirects signaling to Fus3 and allows cells to mate in response to starvation. Mating efficiencies were determined using a quantitative mating assay, and patch assays were done as described (see Methods). Error bars are standard deviations.