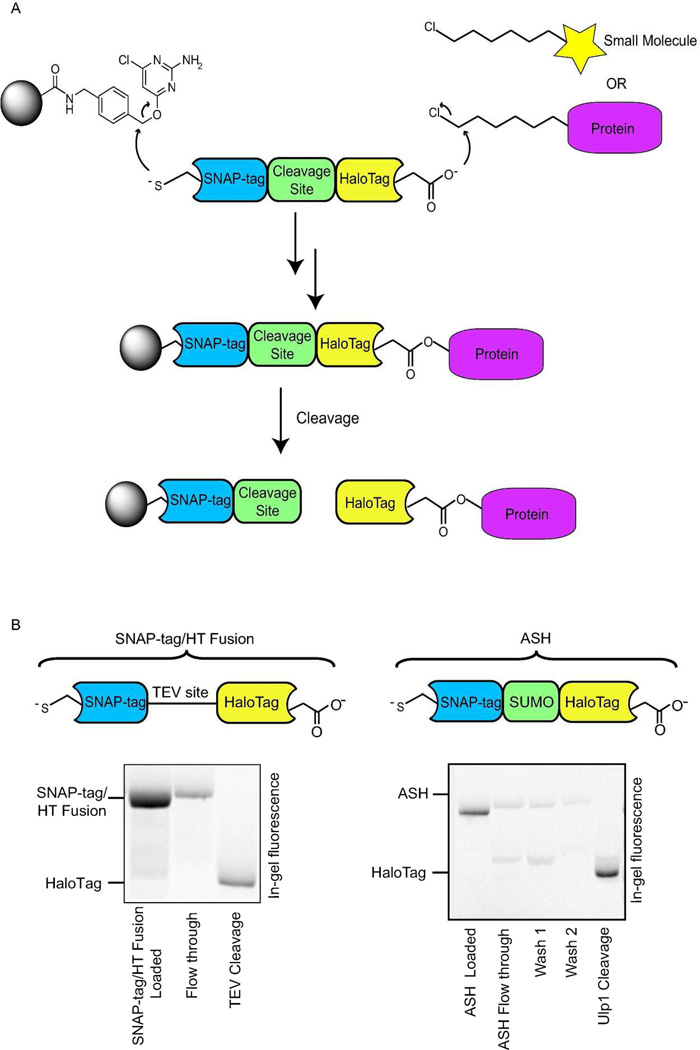
Figure 1.
The hexylchloride-based catch-and-release strategy. a) Overall strategy for the catch-and-release of hexylchloride-labeled small molecules probes and proteins. A protein that contains SNAP-tag fused to HaloTag through a selectively cleavable site serves as a linker between a solid support and captured small molecule probes or proteins. The selective reaction between SNAP-tag and a chloropyrimidine (CLP)-derivatized resin allows immobilization of the bi-functional fusion protein. The support-bound HaloTag enzyme is then able to capture hexylchloride-labeled probes or proteins. Captured proteins are selectively released with a protease that cleaves the linker between SNAP-tag and HaloTag. b) Comparison of the release efficiencies for SNAP-tag/HaloTag fusions that contain either a TEV or SUMO protease cleavage site. A fluorescently-labeled SNAP-tag/HaloTag fusion protein is captured with a CLP-derivatized resin and the fluorophore-modified HaloTag protein is released with a protease. Quantitation of the fluorescence intensities of the flow through and elution fractions allows the efficiency of the capture and release steps to be determined.