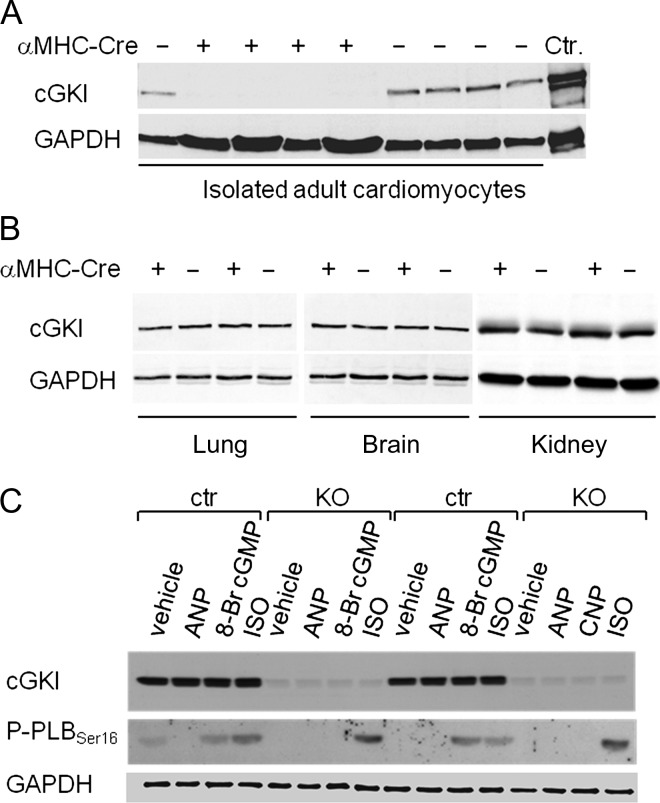
Figure 1.
Expression and activity of cGMP-dependent protein kinase I in cardiomyocytes of control mice and mice with cardiac deletion of cGMP-dependent protein kinase I. (A) cGMP-dependent protein kinase I was detected in isolated cardiomyocytes from control mice but was absent in cardiomyocytes from KO mice (+ αMHC-Cre). (B) cGMP-dependent protein kinase I expression levels in the lung, brain and kidney were not different between genotypes. (C) In cardiomyocytes from control mice, 8-Br cGMP (10 μM, 15 min) and isoproterenol (10 nM, 15 min) increased the phosphorylation of phospholamban at Ser16. In cGMP-dependent protein kinase I-deficient cardiomyocyte (KO), the effect of 8-Br cGMP was abolished but the effect of isoproterenol was preserved. Atrial natriuretic peptide (100 nM, 15 min) did not stimulate phospholamban phosphorylation. GAPDH was used as loading control.