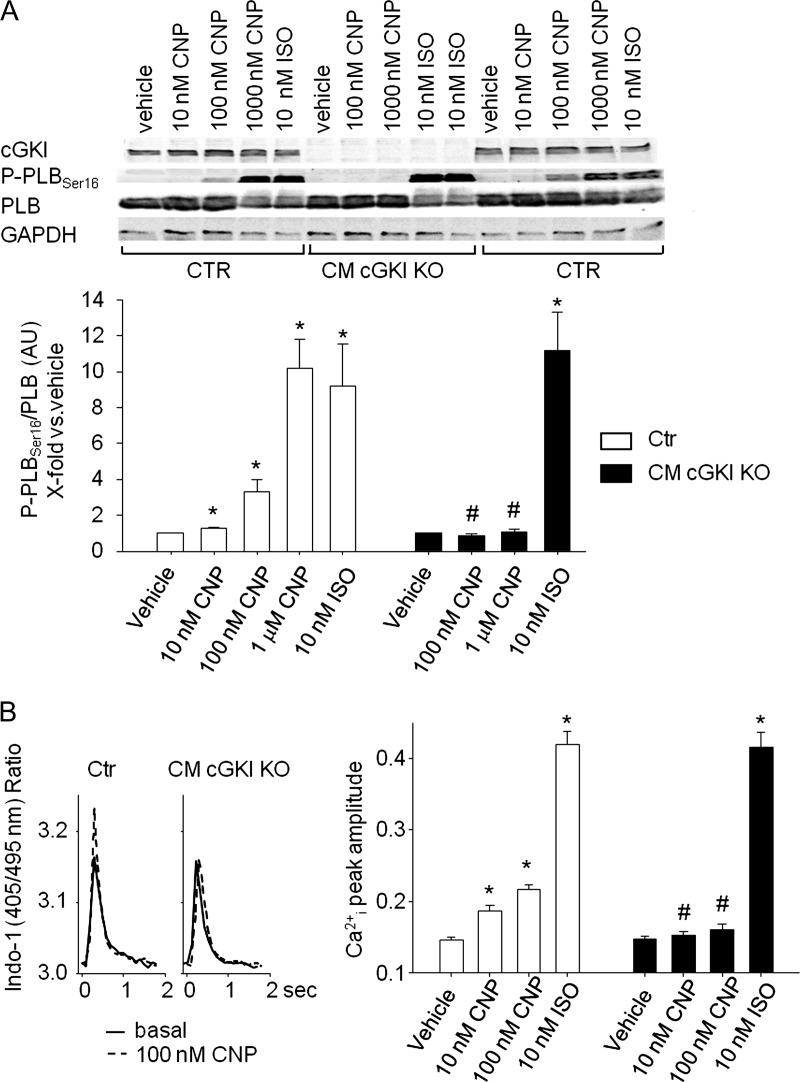
Figure 8.
Effect of CNP on Ser16-phosphorylation of phospholamban and Ca2+-transients of isolated myocytes. (A) Effects of CNP (10 nM–1 μM) and isoproterenol (10 nM, all 15 min) on phospholamban phosphorylation at Ser16 in control and cGMP-dependent protein kinase I-deficient myocytes. (Top) Western blots showing the expression of cGMP-dependent protein kinase I, P-PLBSer16, total phospholamban as well as GAPDH in isolated myocytes. (Bottom) Levels of P-phospholamban were normalized to total phospholamban. Ratios were calculated as x-fold respective vehicle-treated control myocytes. (B, left) Representatives examples of effects of CNP (0.1 μM) on Ca2+ transients in control and cGMP-dependent protein kinase I-deficient myocytes. Panels show respective single traces before (basal) and during CNP treatment. (Right) On average, the peak amplitude of Ca2+-transients (Indo-1 ratio, 405/495 nm, systolic–diastolic) was not different in cGMP-dependent protein kinase I-deficient compared with control myocytes. Superfusion with CNP (10 and 100 nM, 5 min) increased Ca2+ transients in control myocytes. These effects were abolished in cGMP-dependent protein kinase I-deficient cells (n = 8); *P < 0.05 *vs. vehicle, #vs. control.