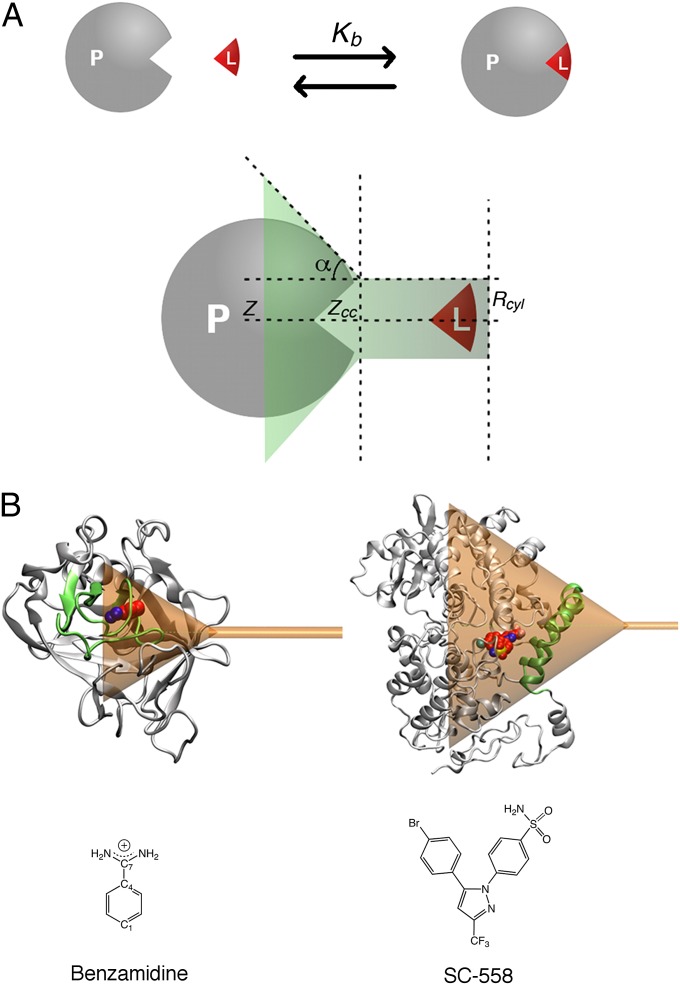
Fig. 1.
(A) Schematic representation of the ligand/protein binding process and the funnel restraint potential used in FM calculations. The shape of the funnel can be customized on the target by setting a few parameters. In particular, given z, the axis defining the exit-binding path of the ligand, zcc is the distance where the restraint potential switches from a cone shape into a cylinder. The α-angle defines the amplitude of the cone, and Rcyl is the radius of the cylindrical section. (B) The funnel restraint potential applied to trypsin (Upper Left) and COX-2 (Upper Right) enzymes with the ligands considered in the study, benzamidine (Lower Left) and SC-558 (Lower Right). In the trypsin case, α is 0.55 rad and zcc is 18 Å (Table S1). In the COX case, α is 0.6 rad and zcc is 44 Å (Table S2). In both cases, Rcyl is set to 1 Å.