Abstract
Immediate postretrieval bilateral blockade of long-acting voltage–dependent calcium channels (L-VDCCs), but not of glutamatergic NMDA receptors, in the dorsal CA1 region of the hippocampus hinders retention of long-term spatial memory in the Morris water maze. Immediate postretrieval bilateral inhibition of calcium/calmodulin-dependent protein kinase (CaMK) II in dorsal CA1 does not affect retention of this task 24 h later but does hinder it 5 d later. These two distinct amnesic effects are abolished if protein degradation by proteasomes is inhibited concomitantly. These results indicate that spatial memory reconsolidation depends on the functionality of L-VDCC in dorsal CA1, that maintenance of subsequent reconsolidated memory trace depends on CaMKII, and these results also suggest that the role played by both L-VDCC and CaMKII is to promote the retrieval-dependent, synaptically localized enhancement of protein synthesis necessary to counteract a retrieval-dependent, synaptic-localized enhancement of protein degradation, which has been described as underlying the characteristic labilization of the memory trace triggered by retrieval. Thus, conceivably, L-VDCC and CaMKII would enhance activity-dependent localized protein renewal, which may account for the improvement of the long-term efficiency of the synapses responsible for the maintenance of reactivated long-term spatial memory.
Memory maintenance or persistence depends on the degree of emotional arousal present at the time of consolidation (1, 2), and apparently on several different biochemical and behavioral variables over the next several hours or days (3–9), especially in the hippocampus, which is widely recognized as the region in charge of consolidation and maintenance (10–13). An important factor in memory maintenance is reconsolidation (14–16), which originates in the labilization of memories caused by nonreinforced retrieval. Reconsolidation is a protein synthesis-dependent mechanism without which traces become progressively weaker (5, 14–19). In most cases, it occurs more readily the first few times that memories are retrieved (3). Otherwise, retrieval triggers extinction, which requires NMDA glutamate receptors and protein synthesis in the hippocampus, the basolateral amygdala, and the ventromedial prefrontal cortex (20–22). The relative predominance of extinction over reconsolidation or vice versa depends on so-called “boundary” conditions, which, aside from the recency of previous training mentioned above, are poorly understood (10, 14, 23–26). Kaang and his coworkers (27–29) have produced evidence of increased proteasome-mediated postsynaptic protein destruction in hippocampus upon retrieval, which would underlie the labilization of the trace at that time (27). Indeed, available evidence suggests that a crucial boundary condition in the extinction/reconsolidation dichotomy may be a balance between synaptic protein degradation and synthesis. The direction of this balance could be regulated by the extracellular-regulated kinase pathway, which, once activated by retrieval (30), participates in extinction but not in reconsolidation (31).
Several of the mechanisms underlying consolidation, reconsolidation, and maintenance depend on intracellular calcium level, [Ca2+]i: for example, the activity of two major protein kinase families, the protein kinase C family (32) and the calcium/calmodulin-dependent kinases (CaMKs). The most important of the latter is α-CaMKII (33–36). An important source of [Ca2+]i are the long-acting voltage-dependent calcium channels (L-VDCCs), which have been proposed to play some role in memory processes (37–40). CaMKII has many purported or demonstrated roles in consolidation and perhaps in maintenance (10); one that has been recently suggested is to serve as a scaffold for proteasomes to act on dendritic spines (41). Several data also suggest a key role for proteasome activity in posttraining memory processing (27–29) up to at least 7 h after consolidation (42).
Here, we study pharmacologically the role of L-VDCCs, proteasomes, and CaMKII on the making, reconsolidation, and maintenance of spatial memories in a Morris water maze. This task has long been known to require the hippocampus (5, 43, 44), particularly through the induction and development of long-term potentiation (LTP) in that structure. Recent studies have extended the requirement of hippocampal LTP to the consolidation of a variety of other learning tasks, spatial and nonspatial (10–12, 22, 45, 46), which widens the scope of studies like the present one for the understanding of memory processes. The mechanisms of hippocampal LTP and memory formation are identical or very nearly so for several memory types (10, 12).
Results
Effect of NMDA Receptor Blockade on Reconsolidation of Long-Term Spatial Memory.
As a major general control experiment, first we determined whether NMDA receptors are required in the hippocampus for reconsolidation of spatial memory in the Morris water maze task (MWM) using a spaced and strong training protocol (47). Rats trained for 5 d were submitted to a probe test in the absence of the escape platform 24 h after the last training session. Immediately after the probe test, the animals received bilateral infusions of vehicle or D(−)-2-amino-5-phosphonopentanoic acid (AP5) (25 nmol per side) in the dorsal hippocampus. Retention was evaluated in a second probe test carried out 24 h or 5 d after the first one. AP5 had no effect on retention when infused into CA1 immediately after the first probe test, regardless of the time elapsed between the two tests (Fig. S1 A–D).
Effect of Blockade of L-VDCCs on Reconsolidation of Long-Term Spatial Memory.
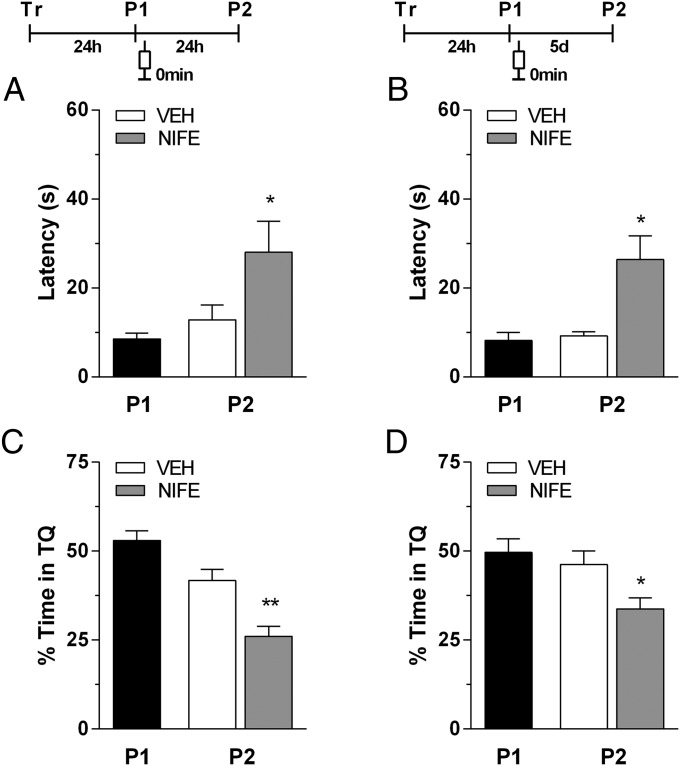
To investigate whether inhibition of L-VDCCs after spatial memory retrieval affects reconsolidation of the reactivated trace, rats trained for 5 d in the spatial version of the MWM as above were submitted to a probe test in the absence of the escape platform 24 h after the last training session. Immediately after the probe test, the animals received bilateral infusions of vehicle or of the L-VDCC blocker nifedipine (NIFE) (10 nmol per side) in the dorsal hippocampus. Retention was evaluated in a second probe test carried out 24 h or 5 d after the first one. When given immediately after the first probe test (P1), NIFE significantly increased the latency to swim over the previous location of the escape platform [t(19) = 2.132, P < 0.05 for P2 24 h after P1 (Fig. 1A); and t(19) = 3.663, P < 0.01 for the second probe test (P2) 5 d after P1 (Fig. 1B)] and reduced to chance level the time spent in the target quadrant during the second probe test, regardless of the time elapsed between the two tests [t(19) =3.625, P < 0.01 for P2 24 h after P1 (Fig. 1C); and t(19) = 2.424, P < 0.05 for P2 5 d after P1 (Fig. 1D)]. Additionally, NIFE did not affect spatial memory when given into dorsal CA1 24 h after the last training session in the absence of a behaviorally relevant event (Fig. S2 A and B) or when administered immediately after a test session in the presence of the escape platform 24 h after training (Fig. S2 C and D).
Fig. 1.
Intrahippocampal infusion of NIFE immediately after nonreinforced retrieval hinders spatial memory retention as measured 24 h or 5 d after reactivation. Animals with infusion cannulae implanted in the CA1 region of the dorsal hippocampus were trained during 5 d in the spatial version of the MWM. Twenty-four hours after the last training session, the animals were randomly assigned to one of four experimental groups and submitted to a 60-s probe test in the absence of the escape platform (P1) (black bar). Immediately after P1, the animals received intrahippocampal infusions of vehicle (VEH) (white bar) or NIFE (10 nmol per side; gray bar). Memory retention was assessed in a second 60-s probe test (P2) carried out 24 h (A and C) or 5 d after P1 (B and D). Data are expressed as means (± SEM) of the latency to swim over the previous location of the escape platform (A and B) or as the percentage of swimming time spent in the target quadrant (TQ) (C and D). *P < 0.05 and **P < 0.01 vs. VEH in Student t test (n = 9–12 per group).
The amnesic effect of NIFE was entirely reversible. In fact, animals that had received intrahippocampal NIFE immediately after P1 acquired the spatial preference to another platform location as consistently as did control animals (Fig. S3).
Effect of Inhibition of CaMKII on Reconsolidation of Long-Term Spatial Memory.
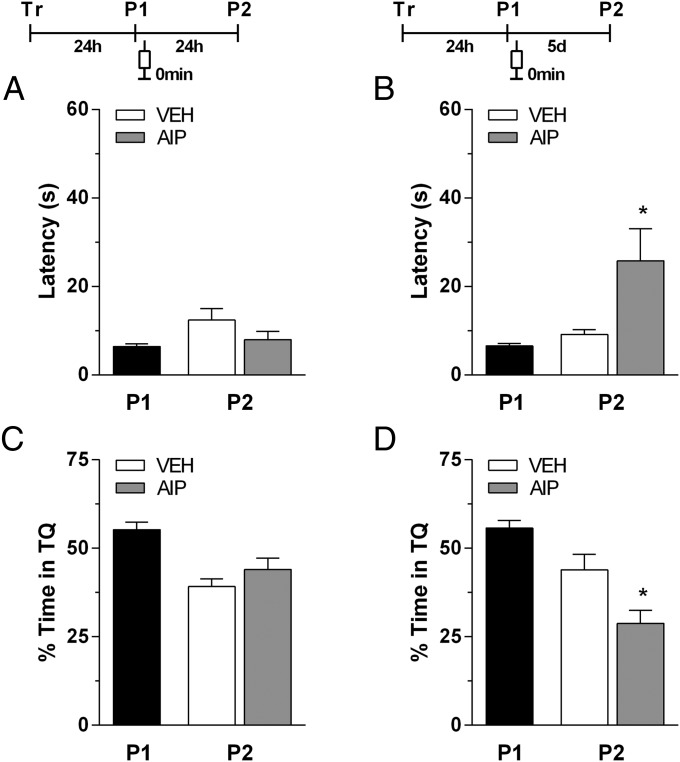
To investigate whether inhibition of CaMKII after spatial memory retrieval affects reconsolidation of the reactivated trace, rats trained for 5 d in the spatial version of the MWM as above were submitted to a probe test in the absence of the escape platform 24 h after the last training session. Immediately, 30 min, or 90 min after the probe test, the animals received bilateral infusions of vehicle or autocamtide-2–related inhibitory peptide (AIP) (1.0 nmol per side) in the dorsal hippocampus. Retention was evaluated in a second probe test carried out 24 h or 5 d after the first one. When given immediately after the first probe test, AIP had no effect on retention if the second probe test was 24 h after the first one (Fig. 2 A and B). However, if the second probe test was 5 d after the first one, AIP caused a significantly increased the latency to swim over the previous location of the escape platform [t(16) = 2.262, P < 0.05 for P2 5 d after P1; Fig. 2C] and reduced to chance level the time spent in the target quadrant during the second probe test [t(16) = 2.627, P < 0.05 for P2 5 d after P1; Fig. 2D]. This result indicates that inhibiting CaMKII immediately after the reactivation of a long-term spatial memory did not hinder the subsequent reconsolidation but, instead, prejudiced the maintenance of the reconsolidated memory, so that this memory trace was no longer retrieved 5 d after the reactivation.
Fig. 2.
Intrahippocampal infusion of AIP immediately after nonreinforced retrieval hinders spatial memory retention only if measured 5 d after reactivation. Animals with infusion cannulae implanted in the CA1 region of the dorsal hippocampus were trained during 5 d in the spatial version of the MWM. Twenty-four hours after the last training session, the animals were randomly assigned to one out of four experimental groups and submitted to a 60-s probe test in the absence of the escape platform (P1) (black bar). Immediately after P1, the animals received intrahippocampal infusions of vehicle (VEH) (white bar) or AIP (1.0 nmol per side; gray bar). Memory retention was assessed in a second 60-s probe test (P2) carried out 24 h (A and C) or 5 d after P1 (B and D). Data are expressed as means (± SEM) of the latency to swim over the previous location of the escape platform (A and B) or as the percentage of swimming time spent in the target quadrant (TQ) (C and D). *P < 0.05 vs. VEH in Student t test (n = 9 per group).
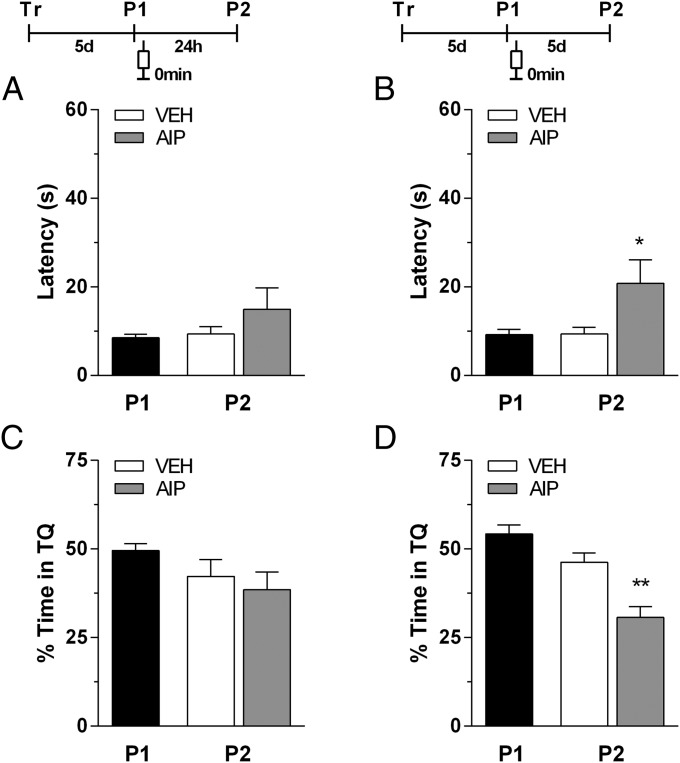
When AIP was given immediately after the first probe test carried out 5 d after the last training session, again, there was no amnesic effect if the second probe test was 24 h after the first one (Fig. 3 A and B), but there was an amnesic effect if a second probe test carried out 5 d after the first one. In this second probe test, AIP significantly increased the latency to swim over the previous location of the escape platform [t(19) = 2.178, P < 0.05 for P2 5 d after P1; Fig. 3C] and reduced to chance level the time spent in the target quadrant during the second probe test [t(19) = 3.880, P < 0.01 for P2 5 d after P1, Fig. 3D]. Thus, this results show that the prejudicial effect of CaMKII inhibition, immediately after the reactivation of a long-term spatial memory, on the maintenance of reconsolidation of this trace memory is independent of how long is the time elapsed between the first probe test and the last training session.
Fig. 3.
Intrahippocampal infusion of AIP immediately after nonreinforced remote retrieval hinders spatial memory retention only if measured 5 d after reactivation. Animals with infusion cannulae implanted in the CA1 region of the dorsal hippocampus were trained during 5 d in the spatial version of the MWM. Five days after the last training session, the animals were randomly assigned to one of four experimental groups and submitted to a 60-s probe test in the absence of the escape platform (P1) (black bar). Immediately after P1, the animals received intrahippocampal infusions of vehicle (VEH) (white bar) or AIP (1.0 nmol per side; gray bar). Memory retention was assessed in a second 60-s probe test (P2) carried out 24 h (A and C) or 5 d after P1 (B and D). Data are expressed as means (± SEM) of the latency to swim over the previous location of the escape platform (A and B) or as the percentage of swimming time spent in the target quadrant (TQ) (C and D). *P < 0.05 and **P < 0.01 vs. vehicle in Student t test (n = 10–12 per group).
There was no effect of AIP, either on reconsolidation or on maintenance of reconsolidated long-term spatial memory, if AIP was bilaterally infused into the dorsal hippocampus 30 min (Fig. S4 A–D) or 90 min after the first probe test (Fig. S4 E–H). Therefore, the effect of AIP on maintenance of reconsolidated long-term spatial memory is restricted to a narrow time window soon after the reactivation of this memory trace.
Finally, AIP did not affect spatial memory when given into dorsal CA1 24 h after the last training session in the absence of a behaviorally relevant event (Fig. S2 A and B) or when administered immediately after a test session in the presence of the escape platform carried out 24 h after training (Fig. S2 C and D).
The amnesic effect of AIP was entirely reversible. In fact, animals that had received intrahippocampal AIP immediately after P1 acquired the spatial preference to another platform location as consistently as did control animals (Fig. S3).
Role of Protein Turnover on the Reconsolidation and Maintenance of Reconsolidated Long-Term Spatial Memory-Respective Dependence on L-VDCC and CaMKII Activities and Protein Synthesis.
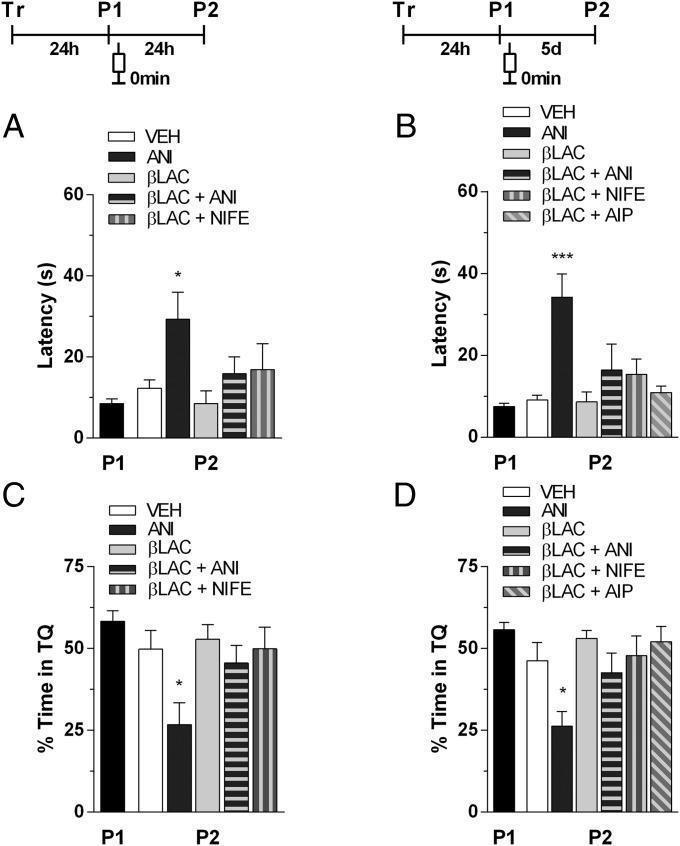
To investigate the role of protein degradation on the effect of blockade of L-VDCC on the long-term spatial memory reconsolidation, on the effect of inhibition of CaMKII activity on the maintenance of the reconsolidated long-term spatial memory, and also on the effect of inhibition of protein synthesis on the long-term spatial memory reconsolidation, rats trained for 5 d in the spatial version of the MWM as above were submitted to a probe test in the absence of the escape platform 24 h after the last training session. Immediately after the probe test, the animals received bilateral infusions of vehicle or anisomycin (ANI) (1.0 μmol per side), clasto-lactacystin β-lactone (βLAC) (200 pmol per side), βLAC plus ANI (200 pmol plus 1.0 μmol per side), βLAC plus NIFE (200 pmol plus 10 nmol per side), or βLAC plus AIP (200 pmol plus 1.0 nmol per side) in the dorsal hippocampus. Retention was evaluated in a second probe test carried out 24 h or 5 d after the first one. When given immediately after the first probe test, ANI significantly increased the latency to swim over the previous location of the escape platform [F(3,28) = 4.305, P < 0.05 for P2 24 h after P1, ANI vs. vehicle (Fig. 4A); and F(3,29) = 7.589, P < 0.001 for P2 5 d after P1, ANI vs. vehicle (Fig. 4B)] and reduced to chance level the time spent in the target quadrant during the second probe test, regardless of the time elapsed between the two tests [F(3,28) = 4.335, P < 0.05 for P2 24 h after P1, ANI vs. vehicle (Fig. 4C); and F(3,29) = 5.455, P < 0.05 for P2 5 d after P1, ANI vs. vehicle (Fig. 4D)]. βLAC, βLAC plus ANI, βLAC plus NIFE, or βLAC plus AIP had no effect on retention when infused into CA1 immediately after the first probe test, regardless of the time elapsed between the two tests (Fig. 4 A–D). Thus, the amnesic effects on reconsolidation caused by blockade of L-VDCC or inhibition of protein synthesis, and on maintenance of reconsolidated long-term spatial memory caused by inhibition of CaMKII, are cancelled by inhibition of proteasome activity. Furthermore, the inhibition of proteasome activity per se is not necessary either for reconsolidation or for maintenance of reconsolidated long-term spatial memory.
Fig. 4.
The amnesic effect induced by the intrahippocampal infusion of ANI, NIFE, or AIP is blocked by proteasome antagonist. Animals with infusion cannulae implanted in the CA1 region of the dorsal hippocampus were trained during 5 d in the spatial version of the MWM. Twenty-four hours after the last training session, the animals were randomly assigned to one out of eleven experimental groups and submitted to a 60-s probe test in the absence of the escape platform (P1) (black bar). Immediately after P1, the animals received intrahippocampal infusions of vehicle (VEH) (white bar), ANI (1.0 μmol per side; dark gray bar), clasto-lactacystin βLAC (200 pmol per side; light gray bar), βLAC plus ANI (200 pmol and 1.0 μmol per side, respectively; horizontally striped bar), βLAC plus NIFE (200 pmol and 10 nmol per side, respectively; vertically striped bar), or βLAC plus AIP (200 pmol and 1.0 nmol per side, respectively; diagonally striped bar). Memory retention was assessed in a second 60-s probe test (P2) carried out 24 h (A and C) or 5 d after P1 (B and D). Data are expressed as means (± SEM) of the latency to swim over the previous location of the escape platform (A and B) or as the percentage of swimming time spent in the target quadrant (TQ) (C and D). *P < 0.05 and ***P < 0.001 vs. vehicle in Dunnett’s test after one-way ANOVA (n = 8–9 per group).
The amnesic effect of ANI was entirely reversible. In fact, animals that had received intrahippocampal ANI immediately after P1 acquired the spatial preference to another platform location as consistently as did control animals (Fig. S3).
Discussion
Memory labilization at the time of retrieval has been explained by proteasome-mediated protein degradation (27), but it also coexists with at least two major protein synthesis-dependent processes: extinction (20, 22) and reconsolidation (14, 15, 23, 48). It is reasonable to think that retrieval triggers or is based upon a reorganization of the synaptic protein structure, where both protein destruction (in the labilization) and construction (in the subsequent reconsolidation) play a role (28, 49). CaMKII, like other signaling pathways [protein kinase C (32), the protein kinase C variant PKMZ (50), and cAMP-dependent protein kinase (13, 51–53)], might be important to signalize the protein synthesis increase and to redirect the newly synthetized proteins to the more active synapses (54–56). This multiple regulation might ensure the use of new proteins for retrieval in the presence of eventually reduced activity of one of the signaling pathways (13, 53). Certainly, several authors (27–29, 49, 57) have proposed that the most likely variable underlying the process of reconsolidation-mediated maintenance is a balance between protein degradation and protein synthesis, rather than any of the two alone. Artinian et al. (48) showed that the inhibition of proteasomes leads to anterograde amnesia; in the present study, it did not. This might be explained by the different task/species combination used by both groups.
The importance of reconsolidation for persistence, and not (necessarily) for further retrieval shortly afterward, is stressed by the facts that several of the drugs studied by postretrieval administration [NIFE (Fig. 1) and AIP (Fig. 2)] showed an effect when the animals were tested 5 d but not just 24 h later. It is likely that mechanisms other than reconsolidation secure retrieval shortly after reconsolidation (3, 10). The present findings attest to the long-lasting nature of reconsolidation effects (17).
Here, we show the link between membrane events and intracellular processes that regulate protein turnover in the maintenance of reconsolidated memory. We demonstrate that this maintenance depends on specific biochemical mechanisms. It is hoped that future research will help to understand this relation. In particular, the factor(s) that trigger proteasomal protein degradation must be better defined.
The present findings might be taken to agree with the recent proposition by Ramachandran and Frey (58) that CaMKII activation, through an influence on actin dynamics during a synaptic capture process such as would accompany a reexposure to water maze learning (22, 46, 55), might play a role in LTP maintenance relevant to spatial memory maintenance. This type of memory is, in fact, the one most traditionally linked to LTP (5, 43).
Recent evidence points to the involvement of different CaMKs in memory processes (55). These enzymes are believed to act by clustering mechanisms that have recently been described (54). The differential timing of these mechanisms and of their linkage to synaptic tagging and capture processes (55) might explain differences in the time course of some of their effects, such as the one on consolidation and early memory maintenance, which is immediate (13, 33), and the one described here on reconsolidation, which is delayed (Fig. 3). The balance between protein synthesis and destruction (42) is activity-dependent (57) and, as discussed above, relies on CaMKII, which redirects proteins to the synapses more active at the time (55). Certainly, the activity that follows after consolidation and after retrieval is different (10, 24). Retrieval induces reconsolidation better 1 or 2 d after consolidation than later (3). Further experiments might elucidate this question.
Materials and Methods
Subjects, Surgery, and Drug-Infusion Procedures.
Three-month-old male Wistar rats weighing 220–280 g and raised in our animal facilities were used in the experiments. Animals were housed four or five to a cage and maintained at 21–23 °C under a 12-h light/12-h dark cycle (lights on at 0700 hours) with free access to food and water. To implant them with indwelling cannulae, rats were deeply anesthetized with 75 mg/kg ketamine (König) plus 10 mg/kg xylazine (Coopers), and 27-gauge 9.0-mm guide cannulae were stereotaxically aimed to the pyramidal cell layer of the dorsal CA1 region, using coordinates (–4.2 anterior, ±3.0 lateral, –2.0 ventral from bregma) taken from the atlas of Paxinos and Watson (59). The animals were allowed to recover from surgery during 4 d before submitting them to any other procedure. At the time of drug delivery, 30-gauge 10.0-mm infusion needles (extending 1.0 mm beyond guide cannulae) were tightly fitted into the guides. Infusions (1.0 μL per side) were carried out over 60 s, and the infusion cannulae were left in place for 30 additional seconds to minimize backflow. Cannulae placement was verified postmortem: 2–4 h after the last behavioral test, 1.0 μL of a 4% (mass/vol) methylene-blue solution was infused as described earlier, and the extension of the dye 30 min thereafter was taken as indicative of the presumable diffusion of the vehicle or drug previously given to each animal. Only data from animals with correct implants were included in the statistical analyses. All experiments were conducted blind to the treatment condition of the animals and following the guidelines of the National Institutes of Health for animal care and use and were approved by the Animal Care and Ethical Committees of the Pontifical Catholic University of Rio Grande do Sul.
Drugs.
AP5, NIFE, clasto-lactacystin βLAC, and ANI were purchased from Sigma, and AIP was obtained from Tocris. AP5 and AIP were first dissolved to working concentration with saline and stored frozen at −20 °C until the moment of use. NIFE and βLAC were first dissolved in 100% DMSO and stored frozen at −20 °C until the moment of use, when they were diluted to working concentration with saline, resulting solutions DMSO 20% in saline. ANI was dissolved in equimolar HCl, diluted with saline and adjusted to pH 7 with NaOH to produce working concentration.
Training in the Spatial Version of the MWM Learning Task.
The water maze was a black circular pool (200 cm in diameter) conceptually divided into four equal imaginary quadrants for the purpose of data analysis. The water temperature was 21–23 °C. Two centimeters beneath the surface of the water and hidden from the rat’s view was a black circular platform (12 cm in diameter). It had a rough surface, which allowed the rats to climb onto it easily once detected. The swimming path of the animals was recorded using a video camera mounted above the center of the pool and analyzed using a video tracking and analysis system. The water maze was located in a well-lit white room with several posters and other distal visual stimuli hanging on the walls to provide spatial cues. Rats were handled 5 min per day for 3 d before training. Training using the spaced training protocol was carried out during 5 successive days (47). On each day, rats received eight consecutive training trials, during which the hidden platform was kept in a constant location. A different starting location was used on each trial, which consisted of a swim followed by a 30-s platform sit. Any rat that did not find the platform within 60 s was guided to it by the experimenter. Memory retention was evaluated in a 60-s probe trial carried out in the absence of the escape platform 24 h or 5 d after the last training session. To evaluate the effect of drugs given after memory reactivation, rats were trained for 5 d as indicated earlier, before being submitted to the first probe test in the absence of the escape platform 24 h or 5 d after the last training session. At different times after that, rats received intra-CA1 infusions of the drug under scrutiny or vehicle. Memory retention was evaluated in a second probe test carried out at 24 or 5 d after the first one.
Statistical Analysis.
Data were analyzed by a two-tailed Student t test or ANOVA followed by Dunnett’s post hoc tests, as appropriate.
Supplementary Material
Acknowledgments
This work supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico and Coordenação para o Aperfeiçoamento do Pessoal de Ensino Superior, both from Brazil (to I.I.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302356110/-/DCSupplemental.
References
- 1.Cahill L, McGaugh JL. A novel demonstration of enhanced memory associated with emotional arousal. Conscious Cogn. 1995;4(4):410–421. doi: 10.1006/ccog.1995.1048. [DOI] [PubMed] [Google Scholar]
- 2.Cahill L, McGaugh JL. Modulation of memory storage. Curr Opin Neurobiol. 1996;6(2):237–242. doi: 10.1016/s0959-4388(96)80078-x. [DOI] [PubMed] [Google Scholar]
- 3.Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36(3):521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 4.Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 5.Wang S-H, Morris RGM. Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol. 2010;61:49–79, C1–C4. doi: 10.1146/annurev.psych.093008.100523. [DOI] [PubMed] [Google Scholar]
- 6.Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. Neuroscientist. 2008;14(2):147–156. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- 7.Eckel-Mahan KL, et al. Circadian oscillation of hippocampal MAPK activity and cAmp: Implications for memory persistence. Nat Neurosci. 2008;11(9):1074–1082. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parfitt GM, Barbosa ÂK, Campos RC, Koth AP, Barros DM. Moderate stress enhances memory persistence: Are adrenergic mechanisms involved? Behav Neurosci. 2012;126(5):729–734. doi: 10.1037/a0029861. [DOI] [PubMed] [Google Scholar]
- 9.Parfitt GM, Campos RC, Barbosa AK, Koth AP, Barros DM. Participation of hippocampal cholinergic system in memory persistence for inhibitory avoidance in rats. Neurobiol Learn Mem. 2012;97(2):183–188. doi: 10.1016/j.nlm.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Izquierdo I, et al. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29(9):496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Gruart A, Muñoz MD, Delgado-García JM. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci. 2006;26(4):1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313(5790):1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 13.Abel T, Nguyen PV. Regulation of hippocampus-dependent memory by cyclic AMP-dependent protein kinase. Prog Brain Res. 2008;169:97–115. doi: 10.1016/S0079-6123(07)00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nader K. Memory traces unbound. Trends Neurosci. 2003;26(2):65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 15.Alberini CM. Mechanisms of memory stabilization: Are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28(1):51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Alberini CM. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front Behav Neurosci. 2011;5(12):1–10. doi: 10.3389/fnbeh.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inda MC, Muravieva EV, Alberini CM. Memory retrieval and the passage of time: From reconsolidation and strengthening to extinction. J Neurosci. 2011;31(5):1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roozendaal B, McGaugh JL. Memory modulation. Behav Neurosci. 2011;125(6):797–824. doi: 10.1037/a0026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudai Y. The restless engram: Consolidations never end. Annu Rev Neurosci. 2012;35:227–247. doi: 10.1146/annurev-neuro-062111-150500. [DOI] [PubMed] [Google Scholar]
- 20.Vianna MR, Szapiro G, McGaugh JL, Medina JH, Izquierdo I. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus. Proc Natl Acad Sci USA. 2001;98(21):12251–12254. doi: 10.1073/pnas.211433298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorenza NG, Rosa J, Izquierdo I, Myskiw JC. Modulation of the extinction of two different fear-motivated tasks in three distinct brain areas. Behav Brain Res. 2012;232(1):210–216. doi: 10.1016/j.bbr.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 22.de Carvalho Myskiw J, Benetti F, Izquierdo I. Behavioral tagging of extinction learning. Proc Natl Acad Sci USA. 2013;110(3):1071–1076. doi: 10.1073/pnas.1220875110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89(1):121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8(4):262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 25.Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5(1):45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- 26.Fioravante D, Byrne JH. Protein degradation and memory formation. Brain Res Bull. 2011;85(1-2):14–20. doi: 10.1016/j.brainresbull.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SH, et al. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science. 2008;319(5867):1253–1256. doi: 10.1126/science.1150541. [DOI] [PubMed] [Google Scholar]
- 28.Kaang BK, Lee SH, Kim H. Synaptic protein degradation as a mechanism in memory reorganization. Neuroscientist. 2009;15(5):430–435. doi: 10.1177/1073858408331374. [DOI] [PubMed] [Google Scholar]
- 29.Kaang BK, Choi JH. Synaptic protein degradation in memory reorganization. Adv Exp Med Biol. 2012;970:221–240. doi: 10.1007/978-3-7091-0932-8_10. [DOI] [PubMed] [Google Scholar]
- 30.Barros DM, et al. Molecular signalling pathways in the cerebral cortex are required for retrieval of one-trial avoidance learning in rats. Behav Brain Res. 2000;114(1-2):183–192. doi: 10.1016/s0166-4328(00)00226-6. [DOI] [PubMed] [Google Scholar]
- 31.Fischer A, et al. Hippocampal Mek/Erk signaling mediates extinction of contextual freezing behavior. Neurobiol Learn Mem. 2007;87(1):149–158. doi: 10.1016/j.nlm.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonini JS, et al. On the participation of hippocampal PKC in acquisition, consolidation and reconsolidation of spatial memory. Neuroscience. 2007;147(1):37–45. doi: 10.1016/j.neuroscience.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Wolfman C, et al. Intrahippocampal or intraamygdala infusion of KN62, a specific inhibitor of calcium/calmodulin-dependent protein kinase II, causes retrograde amnesia in the rat. Behav Neural Biol. 1994;61(3):203–205. doi: 10.1016/s0163-1047(05)80001-9. [DOI] [PubMed] [Google Scholar]
- 34.Mayford M, et al. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274(5293):1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 35.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13(3):169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coultrap SJ, Bayer KU. CaMKII regulation in information processing and storage. Trends Neurosci. 2012;35(10):607–618. doi: 10.1016/j.tins.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Power JM, Sah P. Intracellular calcium store filling by an L-type calcium current in the basolateral amygdala at subthreshold membrane potentials. J Physiol. 2005;562(Pt 2):439–453. doi: 10.1113/jphysiol.2004.076711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fourcaudot E, et al. L-type voltage-dependent Ca(2+) channels mediate expression of presynaptic LTP in amygdala. Nat Neurosci. 2009;12(9):1093–1095. doi: 10.1038/nn.2378. [DOI] [PubMed] [Google Scholar]
- 39.Kochlamazashvili G, et al. The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca(2+) channels. Neuron. 2010;67(1):116–128. doi: 10.1016/j.neuron.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perugini A, Laing M, Berretta N, Aicardi G, Bashir ZI. Synaptic plasticity from amygdala to perirhinal cortex: A possible mechanism for emotional enhancement of visual recognition memory? Eur J Neurosci. 2012;36(4):2421–2427. doi: 10.1111/j.1460-9568.2012.08146.x. [DOI] [PubMed] [Google Scholar]
- 41.Bingol B, et al. Autophosphorylated CaMKIIalpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell. 2010;140(4):567–578. doi: 10.1016/j.cell.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Salon M, et al. The ubiquitin-proteasome cascade is required for mammalian long-term memory formation. Eur J Neurosci. 2001;14(11):1820–1826. doi: 10.1046/j.0953-816x.2001.01806.x. [DOI] [PubMed] [Google Scholar]
- 43.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 44.Martin SJ, Clark RE. The rodent hippocampus and spatial memory: From synapses to systems. Cell Mol Life Sci. 2007;64(4):401–431. doi: 10.1007/s00018-007-6336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clarke JR, Cammarota M, Gruart A, Izquierdo I, Delgado-García JM. Plastic modifications induced by object recognition memory processing. Proc Natl Acad Sci USA. 2010;107(6):2652–2657. doi: 10.1073/pnas.0915059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Almaguer-Melian W, et al. Novelty exposure overcomes foot shock-induced spatial-memory impairment by processes of synaptic-tagging in rats. Proc Natl Acad Sci USA. 2012;109(3):953–958. doi: 10.1073/pnas.1114198109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Da Silva WC, et al. Inhibition of mRNA synthesis in the hippocampus impairs consolidation and reconsolidation of spatial memory. Hippocampus. 2008;18(1):29–39. doi: 10.1002/hipo.20362. [DOI] [PubMed] [Google Scholar]
- 48.Artinian J, et al. Protein degradation, as with protein synthesis, is required during not only long-term spatial memory consolidation but also reconsolidation. Eur J Neurosci. 2008;27(11):3009–3019. doi: 10.1111/j.1460-9568.2008.06262.x. [DOI] [PubMed] [Google Scholar]
- 49.Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6(3):231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- 50.Sacktor TC. How does PKMζ maintain long-term memory? Nat Rev Neurosci. 2011;12(1):9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- 51.Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260(5114):1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 52.Bernabeu R, et al. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci USA. 1997;94(13):7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Izquierdo LA, et al. Different hippocampal molecular requirements for short- and long-term retrieval of one-trial avoidance learning. Behav Brain Res. 2000;111(1-2):93–98. doi: 10.1016/s0166-4328(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 54.Hudmon A, et al. A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. J Neurosci. 2005;25(30):6971–6983. doi: 10.1523/JNEUROSCI.4698-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Redondo RL, et al. Synaptic tagging and capture: Differential role of distinct calcium/calmodulin kinases in protein synthesis-dependent long-term potentiation. J Neurosci. 2010;30(14):4981–4989. doi: 10.1523/JNEUROSCI.3140-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sajikumar S, Navakkode S, Frey JU. Identification of compartment- and process-specific molecules required for “synaptic tagging” during long-term potentiation and long-term depression in hippocampal CA1. J Neurosci. 2007;27(19):5068–5080. doi: 10.1523/JNEUROSCI.4940-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fonseca R, Vabulas RM, Hartl FU, Bonhoeffer T, Nägerl UV. A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron. 2006;52(2):239–245. doi: 10.1016/j.neuron.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 58.Ramachandran B, Frey JU. Interfering with the actin network and its effect on long-term potentiation and synaptic tagging in hippocampal CA1 neurons in slices in vitro. J Neurosci. 2009;29(39):12167–12173. doi: 10.1523/JNEUROSCI.2045-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Compact 3rd Ed, Vol 1. San Diego: Academic; 1997. pp. 33–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.