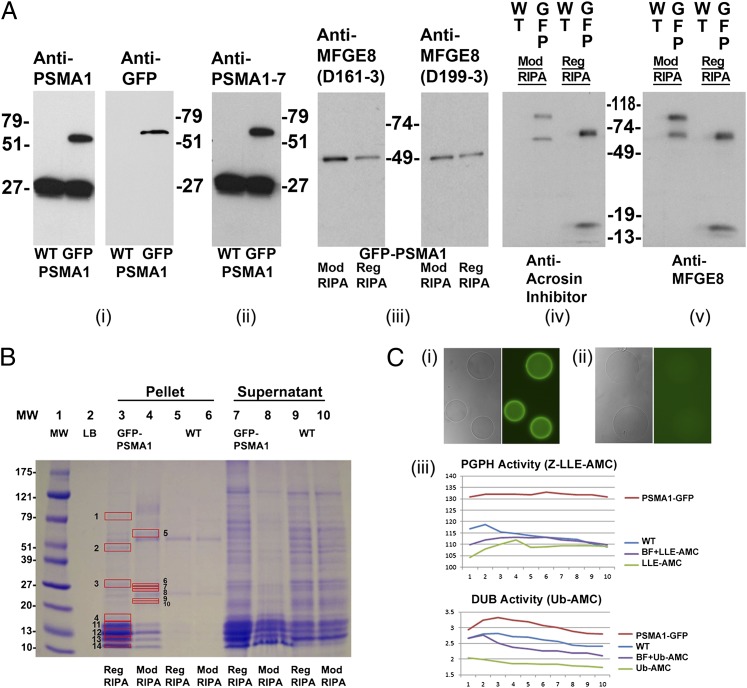
Fig. 4.
Proteomic analysis of PSMA1-GFP fusion protein and the coimmunoprecipitated proteins in the transgenic spermatozoa. (A) A unique band of ∼57 kDa, corresponding to the calculated mass of PSMA1-GFP, is detected in the sperm extracts of the transgenic boar (GFP lane), but not in those from a wild-type boar (WT lane) (A, i). Anti-PSMA1 antibody detected both the wild-type PSMA1 band of ∼27 kDa and the PSMA1-GFP fusion protein of ∼57 kDa (A, i, Left), whereas the anti-GFP antibody detected the PSMA1-GFP band in the transgenic boar only (A, i, Right). A polyclonal antibody recognizing six of seven 20S proteasomal core α-type/PSMA subunits confirms the presence of PSMA1-GFP in the transgenic boar and of the six wild-type PSMA subunits, all of which comigrate in the 25–30-kDa range in both WT and transgenic spermatozoa (A, ii). Western blotting was used to confirm the coimmunoprecipitation of lactadherin MFGE8 with sperm proteasomes from transgenic sperm extracts, using two different anti-MFGE8 antibodies, D161-3 and D199-3 (A, iii). Spermatozoa were lysed with regular RIPA buffer (Reg RIPA) or modified RIPA buffer (Mod RIPA), immunoprecipitated with anti-GFP antibodies, and probed with anti-MFGE8 antibodies. A single 51-kDa band was revealed in each lane and corresponds to the calculated mass of MFGE8. A modified RIPA buffer (Nonidet P-40) was included because the regular RIPA buffer (Triton X-100) may disrupt some protein–protein interactions. A cross-precipitation experiment was performed to confirm the interaction of MFGE8 (one of the identified interacting proteins from the proteomics results shown in B) with GFP-tagged proteasomes. Wild-type and transgenic boar spermatozoa were lysed with regular or modified RIPA buffer and immunoprecipitated with anti-MFGE8 antibodies (A, iv). The precipitations then were probed with anti-GFP antibodies. Bands were revealed in all transgenic boar lanes, with no bands detected in the wild-type lanes. (B) Band patterns of the putative proteasome-interacting proteins coimmunoprecipitated from the transgenic boar spermatozoa by using an anti-GFP antibody. Wild-type boar and transgenic boar spermatozoa were solubilized with RIPA or modified RIPA buffer and then immunoprecipitated with anti-GFP antibodies. Red boxes mark proteins unique to transgenic boar sperm that were analyzed by MS. Protein numbers correspond to the protein numbers in SI Appendix, Table 5B. (C) Affinity purification of the enzymatically active green fluorescent proteasomes. The GFP purification matrix beads show high fluorescence intensity after incubation with the PSMA1-GFP sperm extracts (C, i); there is no fluorescence in the beads incubated with the wild-type sperm extract (C, ii). Both types of beads were photographed at an identical magnification and acquisition time. (C, iii) Proteasomal PGPH activity (Upper; measured using the fluorometric proteasomal substrate Z-LLE-AMC) and deubiquitinating activity [Lower; measured by fluorometric substrate ubiquitin (Ub)-AMC] in the eluted fraction from beads incubated with PSMA1-GFP sperm extracts (PSMA1-GFP), in the eluted fraction from beads incubated with wild-type sperm extract (WT), in the assay solution including elution buffer and fluorometric substrate (BF+LLE-AMC or BF+Ub-AMC), or in the assay solution containing only the fluorometric substrate (Z-LLE-AMC or Ub-AMC). Proteasomal activities were measured every 2 min for 20 min.