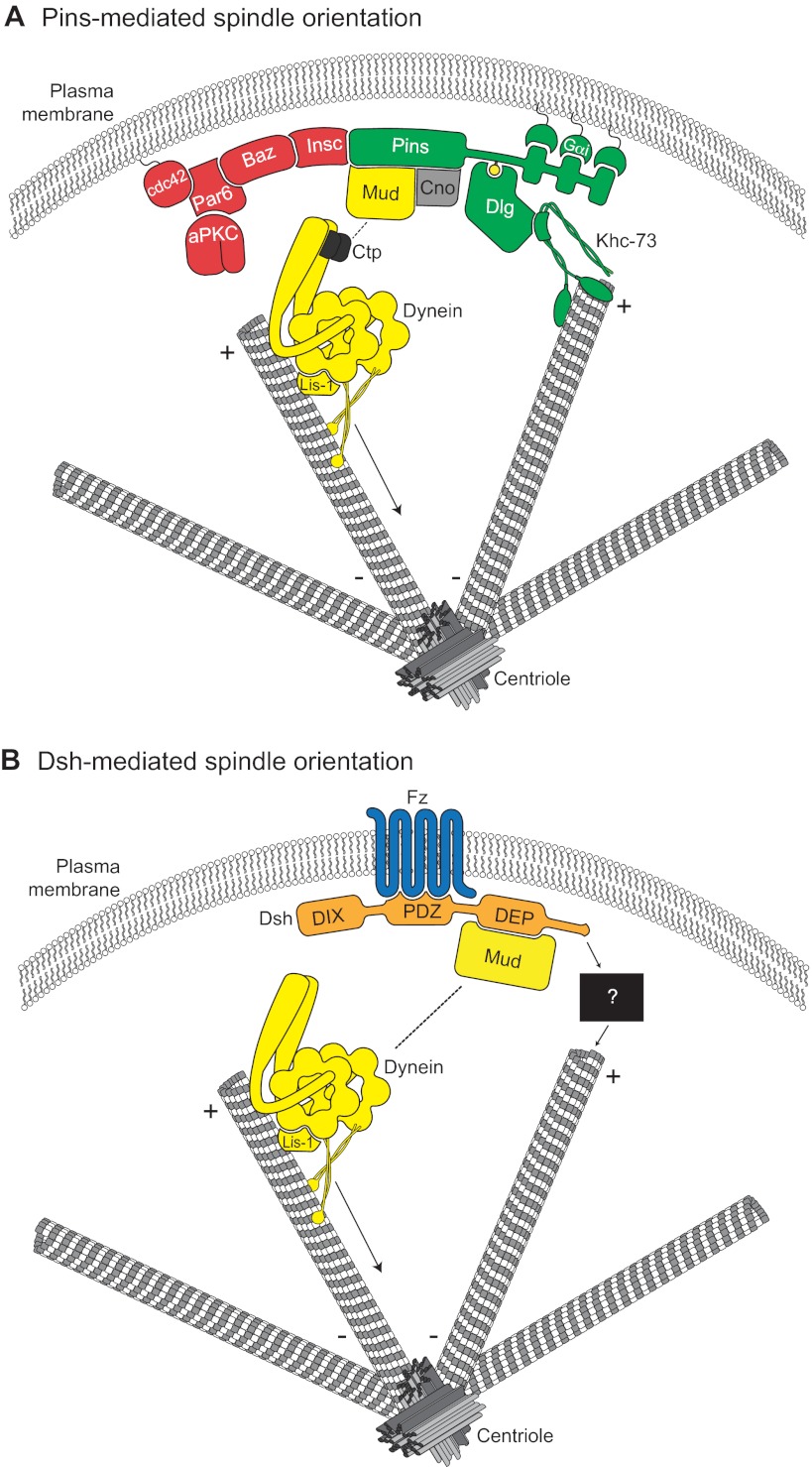
Fig. 3.
Molecular models for Pins- and Dsh-mediated spindle orientation. (A) The Cdc42-Par6-aPKC-Baz polarity complex associates with Pins through the adapter protein Insc. Pins cortical association is mediated by direct binding between three consecutive GoLoco domains and the heterotrimeric G-protein Gαi. Pins serves as the ‘hub’ for two distinct spindle orientation pathways in Drosophila neuroblasts. First, Pins (via its TPR domain, not shown) binds directly to Mud, which associates with cytoplasmic dynein through Ctp. Lis-1 functions as an activator of dynein microtubule motor activity. The scaffold protein Cno is required for maintenance of the apical Pins-Mud complex. The dynein-dynactin complex moves processively towards MT minus-ends (arrow) and generates pulling forces on the mitotic spindle. Second, the Pins linker domain is phosphorylated (yellow circle) by Aurora-A kinase. This phosphorylation allows direct binding with Dlg, which then associates with the kinesin motor protein Khc-73; the plus-end binding capacity of Khc-73 is thought to provide an MT capture or attachment mechanism. Together, the MT-capture and force-generation pathways elicit robust spindle alignment. (B) The Frizzled receptor (Fz, blue) is planar polarized in many cell types. Following Wnt activation, Fz recruits the cytoplasmic scaffold protein Dishevelled (Dsh, orange) to the cortex via its PDZ domain. The Fz-Dsh complex regulates spindle orientation in Drosophila sensory organ precursor cells through association (via the Dsh DEP domain) with Mud. Similar to Pins, Mud associates with the dynein motor complex. Whether the Dsh-Mud pathway is sufficient for spindle orientation or if additional pathways (black box) are required remains to be investigated.