Figure 2.
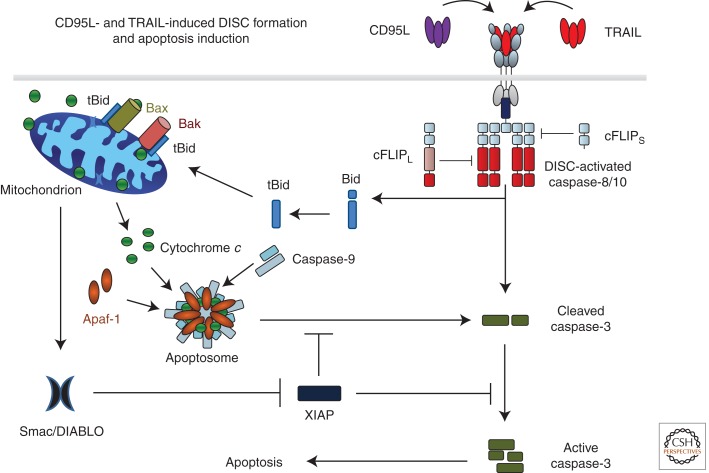
CD95L- and TRAIL-induced DISC formation and apoptosis induction. Binding of CD95L or TRAIL to their cognate receptors leads to receptor trimerization and formation of the death-inducing signaling complex (DISC). The DD of one FADD molecule interacts with the DDs of the three cross-linked receptors. Subsequently, procaspases 8 and 10 are recruited by interaction of their DED with that of FADD. Heterodimers between cFLIPL and caspase-8 or -10 are not inactive as proteolytic enzymes but their proteolytic activity is edited as compared with caspase-8 or -10 homodimers. cFLIPS in turn inhibits caspase activity at the DISC by preventing dimerization of caspase-8/10/cFLIPL. Interestingly, one FADD molecule recruits six to 10 DED-containing caspase-8, -10, and cFLIP proteins. DISC-activated caspase-8/-10 cleaves caspase-3, enabling autoactivation of caspase-3, which renders the enzyme fully active. This latter step can, however, be blocked by X-linked inhibitor of apoptosis protein (XIAP). DISC-activated caspase-8/-10 also cleaves Bid (tBID). Interaction of tBid with Bak and Bax in the mitochondrial outer membrane induces Bax/Bak oligomerization, resulting in mitochondrial outer-membrane permeabilization (MOMP) so that cytochrome c and Smac/DIABLO are released from the mitochondrial intermembrane space to the cytosol. Cytochrome c, together with Apaf-1 and caspase-9, forms the apoptosome, the activation platform for caspase-9, which can, however, also be inhibited by XIAP. Smac/DIABLO binds to XIAP, which releases caspase-3 and -9 from XIAP-imposed inhibition. Activation of these caspases enables execution of apoptotic cell death.