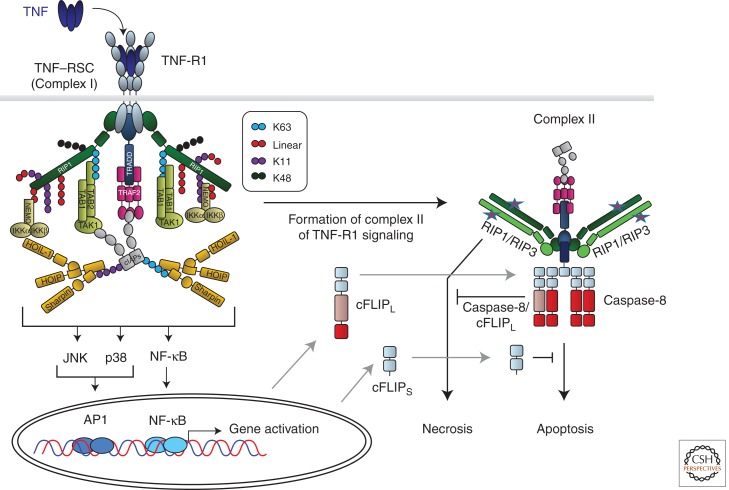
Figure 3.
TNF-R1-induced gene activation and cell death signaling. Cross-linking of TNF-R1 by TNF results in formation of the TNF-R1 signaling complex (TNF–RSC). Cross-linked TNF-R1 recruits TRADD and RIP1 to the DD of the receptor. Subsequently, TRADD recruits TRAF2, which in turn provides the platform for cIAP1/2. cIAPs then place ubiquitin chains, linked via different interubiquitin linkages, on various TNF–RSC components. cIAP-Mediated ubiquitination is required to recruit LUBAC. Once recruited, LUBAC places linearly linked ubiquitin linkages on RIP1 and NEMO. Together, the different cIAP- and LUBAC-generated ubiquitin chains, placed in defined positions and sequences on specific components of the TNF–RSC, enable the physiologically required gene-activatory capacity of this complex by mediating the exact positioning of both the IKK and TAB/TAK complexes in the TNF–RSC. The different ubiquitin linkages are indicated in different colors. The depicted chain lengths, the sequence of the different linkages in them, and their exact positioning on different TNF–RSC components are only shown as examples in this model of the TNF–RSC as they are currently mostly unknown. Most likely involving the action of deubiquitinases (DUBs; not depicted here), the TNF–RSC releases TRADD, together with RIP1 and other cytoplasmic constituents of the complex, into the cytosol. This secondary complex, complex II, recruits FADD, caspase-8/10, and, when expressed, the different isoforms of cFLIP and RIP3. RIP1/3-induced necrosis from complex II is counteracted by the activity of the caspase-8/cFLIPL heteromer, and FADD/caspase-8-mediated apoptosis by cFLIPS and possibly also by cFLIPL. Depending on the relative presence of the components in complex II, it can therefore either initiate FADD/caspase-8-dependent apoptosis or RIP1/RIP3-kinase-activity-dependent necrosis, or, when cFLIP and perhaps other, currently unknown inhibitory factors are present in the complex at sufficiently high levels, its cell-death-inducing capacity may be entirely inhibited.