Figure 2.
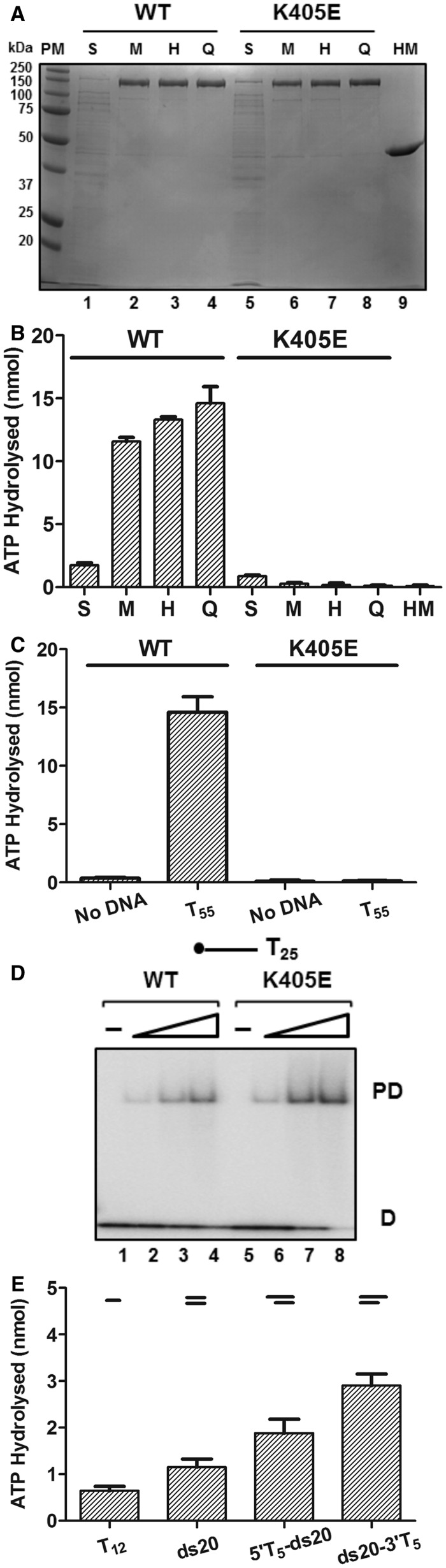
Biochemical analysis of D2. (A) A 12% SDS-PAGE analysis of His-MBP-D2 WT (lanes 1–4) and K405E mutant (lanes 5–8) proteins at each purification step: supernatant from bacterial lysate (S), eluate from amylose column (M), eluate from HisTrapTM column (H), eluate from Source Q column (Q); His-MBP tag protein (HM) control; 1 μg total protein. (B) The ATPase activity of His-MBP-D2 WT and K405E protein samples from each purification step and His-MBP tag (74.5 ng total protein per assay) was determined in the presence of oligonucleotide T55 (25 nM). The experiment was repeated three times [n = 3, mean and standard deviation (SD)]. (C) ATPase activities of D2 WT and K405E (25 nM) were determined in the absence or presence of T55 (25 nM). The value for ATP hydrolysed at 15 min is given. Further detailed characterization of the ATPase activity of purified D2 indicated that the rate of ATP hydrolysis under the standard assay conditions is outside the linear range after 20 min (Supplementary Figure S3). (D) Binding of D2 WT and K405E (Lanes 2–4 and 6–8, 0.01, 0.05 and 0.1 nM protein) to 5′ 32P-end-labelled T25 ssDNA (0.05 nM substrate) determined by EMSA and visualized by phosphorimaging (PD, protein–DNA complex; D, free DNA). A small increase in ssDNA-binding activity was observed repeatedly with D2 K405E. (E) ATPase activity of D2 WT (25 nM) measured in the presence of various DNA substrates (25 nM): ssDNA, T12; blunt-ended dsDNA, ds20; partial duplexes with a 20 bp and a 5′ or 3′ 5-base oligo-dT overhang, 5′T5-ds20 and ds20-3′T5.