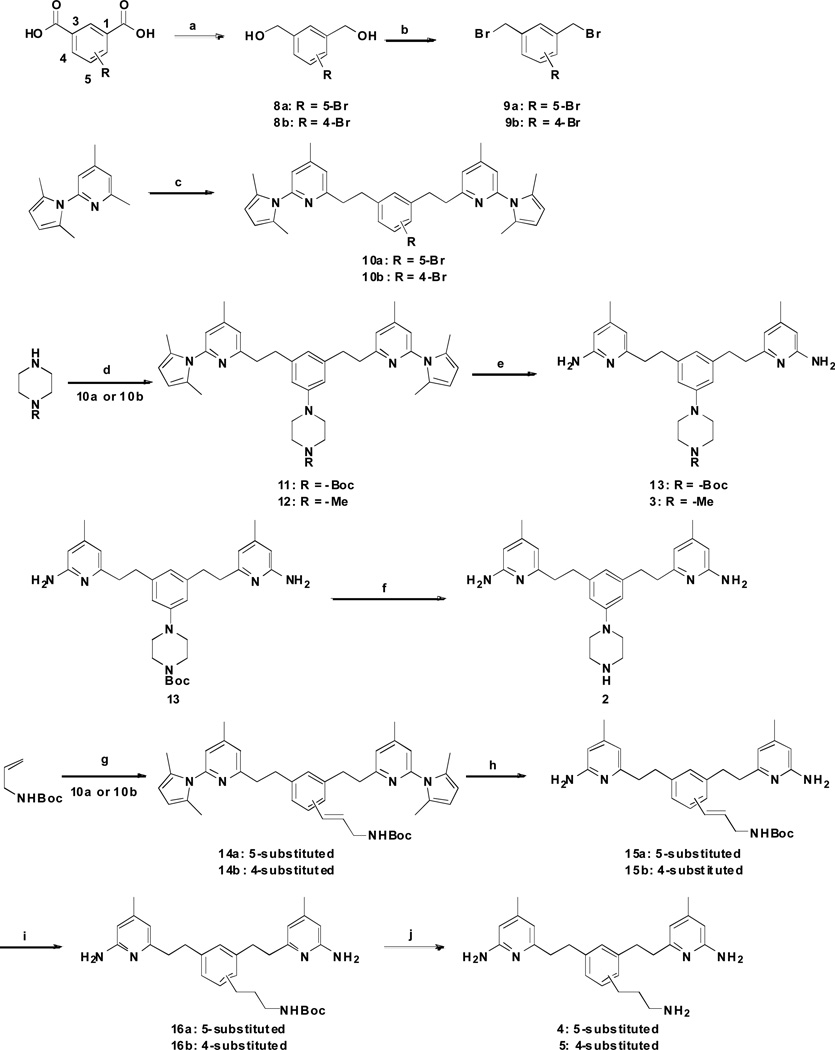
Scheme 1.
Synthesis of 2–5a
a Reagents and conditions: (a) LiBH4, TMSCl, THF, rt, 12 h, 82–86%; (b) PPh3, CBr4, CH2Cl2, 0 °C, 2 h, 89–92%; (c) 9a or 9b, n-BuLi, THF, −78 °C to rt, 2 h, 48–56%; (d) Pd2(dba)3, BINAP, NaOtBu, PhMe, 80 °C, 8 h, 61–67 %; (e) NH2OH·HCl, EtOH/H2O (2:1), 100 °C, 36 h, 58–69%; (f) 3N HCl/MeOH, rt, 24 h, quantitative; (g) PdCl2, DIEA, Tri(o-tolyl)phosphine, DMF, 120 °C, 8 h, 58–64%; (h) NH2OH·HCl, EtOH/H2O (2:1), 100 °C, 36 h, 49–56%; (i) H2, Pd/C, MeOH, rt, 12 h, quantitative; (j) 3N HCl/MeOH, rt, 24 h, quantitative.