Fig. 1.
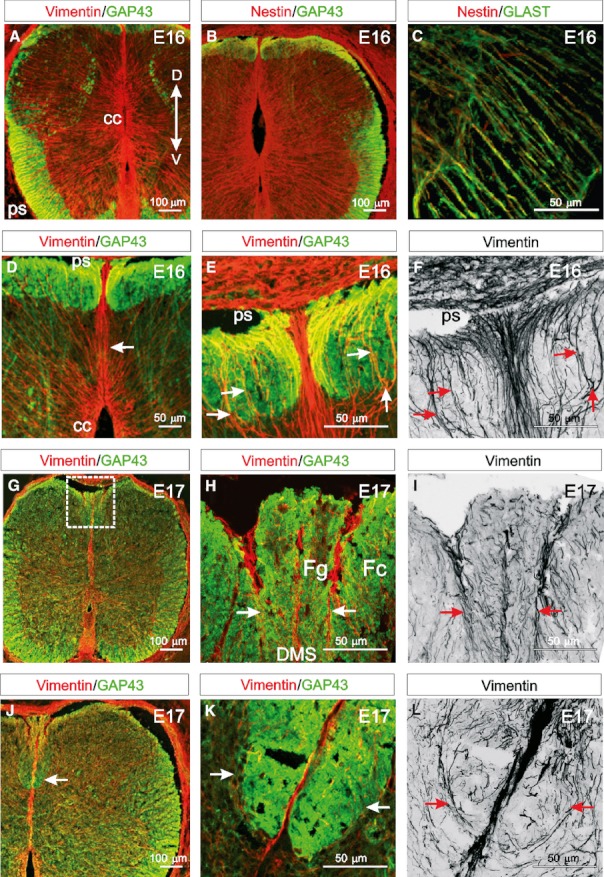
DMS-fibres facilitate the formation of the dorsal funiculi. (A,B) At E16 vimentin- and nestin-immunoreactive radial glial cells are present in dorsal and ventral regions of the spinal cord extending from the central canal (cc) to the pial surface (ps). GAP43 is evident in the growing ventral, lateral, dorsolateral and dorsal funiculi. (C) Nestin is coexpressed with the glial specific marker GLAST in radial glial cell processes. (D) At E16, the vimentin-immunoreactive DMS extends from the dorsal central canal to the dorsal pial surface and divides the emerging GAP43-immunoreactive dorsal funiculi bilaterally. (E) Vimentin-immunoreactive collaterals (DMS-fibres) emanating from the DMS appear to provide a scaffold around which the dorsal funiculi grow (arrows). (F) Panel (E) converted to grey-scale, which clearly highlights vimentin-immunoreactive processes emanating laterally from the DMS on both sides of the spinal cord and then extending dorsally (arrows) to the pial surface. (G) Early at E17, the GAP43-immunoreactive cuneate and gracile fasciculi begin to separate in the dorsal spinal cord (box). (H) A higher magnification image of a region similar to that boxed in (J) showing vimentin-immunoreactive DMS-fibres appearing to segregate the more medial gracile fasciculus (Fg) from the cuneate fasciculus (Fc) (arrows). (I) Panel (H) converted to grey-scale, highlighting how fascicles of vimentin-immunoreactive DMS-fibres extend from the DMS to the dorsal pial surface (arrows). (J) At late E17, the cuneate fasciculus enlarges ventrally (arrow). (K) A higher magnification image of a similar region indicated by the arrow in (J) showing a band of vimentin-immunoreactive processes radiating from the DMS bilaterally to surround the growing cuneate fasciculus, seemingly forming a framework through which axons grow (arrows). (L) Panel (K) converted to grey-scale, showing vimentin-immunoreactive DMS-fibres extending laterally from the DMS first then extending dorsally (arrows). (Images A–C, D, G and J are confocal projections of 15–20 images captured at 0.5-μm intervals. Images E, F, H–I and K and L are confocal projections of 65–70 images captured at 0.5-μm intervals. Scale bars are in microns as indicated. Dorsoventral orientations are consistent for all images as indicated in A).
