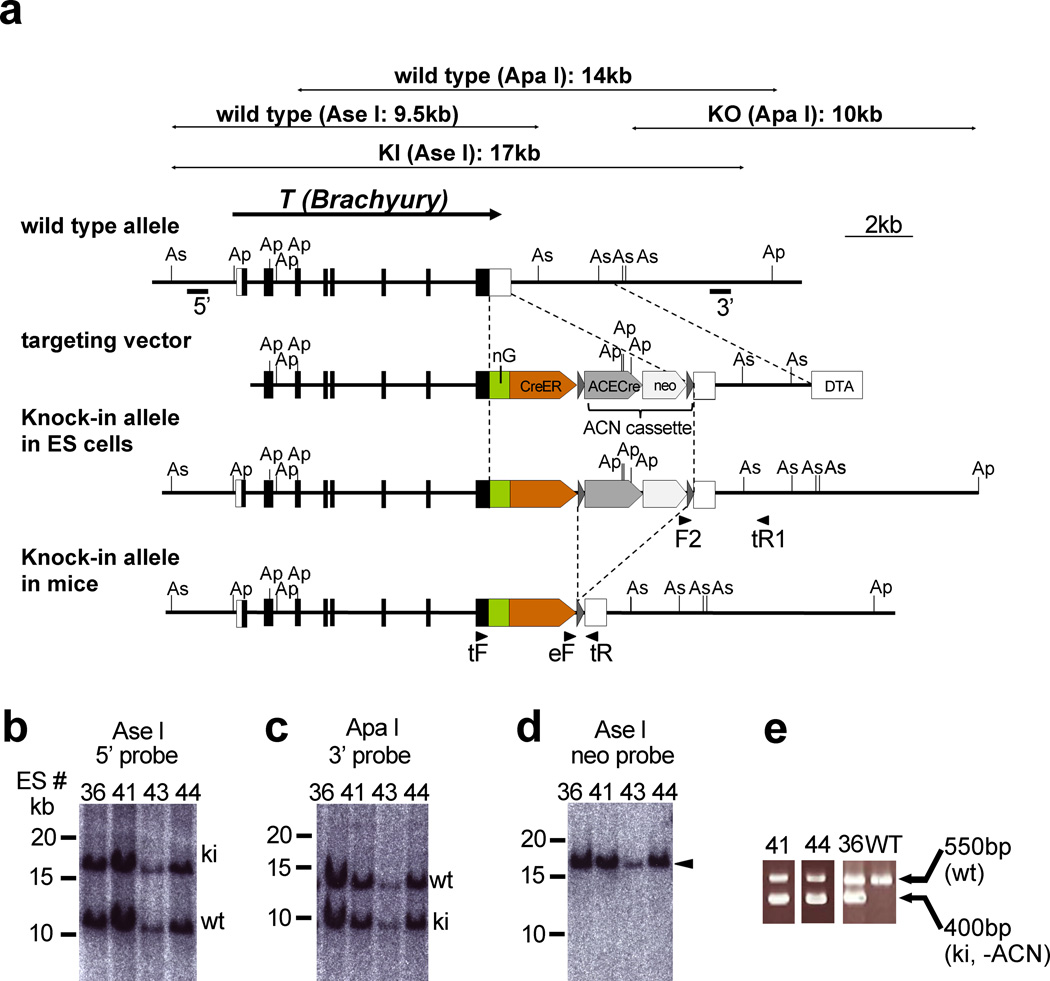
Figure 2. Production of the TnEGFP-CreERT2/+ mouse.
(a) Targeting strategy. The TnEGFP-CreERT2 allele was generated by homologous recombination in ES cells. Exons are indicated by filled and open boxes, and coding regions by filled boxes. Triangles represent loxP sites. 5’ and 3’ indicate the approximate positions of the 5’ and 3’ probes, respectively, for Southern hybridization. Abbreviations: As, Ase I; Ap, Apa I; nEGFP, ERAV 2A peptide followed by nuclear (histone H2B) EGFP; CreER, TaV 2A peptide followed by CreERT2; ACE-Cre, a cassette carrying the testis-specific ACE promoter-Cre-poly A signal; neo, an expression cassette for the neomycin-resistance gene; DT-A, MC1-DT-A-poly A signal cassette. The ACN cassette consists of the tACE-Cre and neo cassettes flanked by loxP sites. Arrowheads indicate the approximate positions of the PCR primers. F2, SVloxP-3’-F2; tR1, T-PC-3’-R1; tF, T genotype F; eF, ERT2 genotype F; tR, T genotype R.
(b–d) Southern blot analysis of homologous recombination in ES cells. Ase I-digested (b, d) or Apa I-digested (c) ES cell genomes were separated on a 0.3% agarose gel and blotted onto nylon membranes. Hybridization with the 5’ probe (b) and the 3’ probe (c) yielded two bands that corresponded to the predicted lengths of the wild-type (wt) and knock-in (ki) alleles. Hybridization with the internal (neo) probe (d) gave a single band (arrowhead) corresponding to the knock-in allele. (e) Genotype determination of TnEGFP-CreERT2 mice by PCR. Note that the ACN cassette was removed in knock-in mice #36, 41 and 44.