Abstract
Transmission of Mycobacterium tuberculosis (Mtb) continues uninterrupted. Pre-exposure vaccination remains a central focus of tuberculosis research but 25 years of follow up is needed to determine whether a novel childhood vaccination regime protects from adult disease, or like BCG assists Mtb dissemination by preventing childhood illness but not infective adult pulmonary tuberculosis. Therefore, different strategies to interrupt the life cycle of Mtb need to be explored. This personal perspective discusses alternative approaches that may be delivered in a shorter time frame.
INTRODUCTION
It is almost 20 years now since the WHO declared tuberculosis (TB) a global health pandemic, and this rallying call has led to significant investment in research over that last 2 decades. However, despite the great advances in our understanding of TB biology, Mycobacterium tuberculosis (Mtb) remains depressingly successful as a global pathogen (1). Furthermore, with the emergence of multidrug resistant drug and extensively drug-resistant disease (2), TB cases are becoming increasingly hard and expensive to treat, and the spectre of totally-drug resistant disease has emerged (3). Consequently, perhaps we need to re-consider where the infectious cycle can most effectively be interrupted.
WHERE IS THE MTB LIFE CYCLE VULNERABLE?
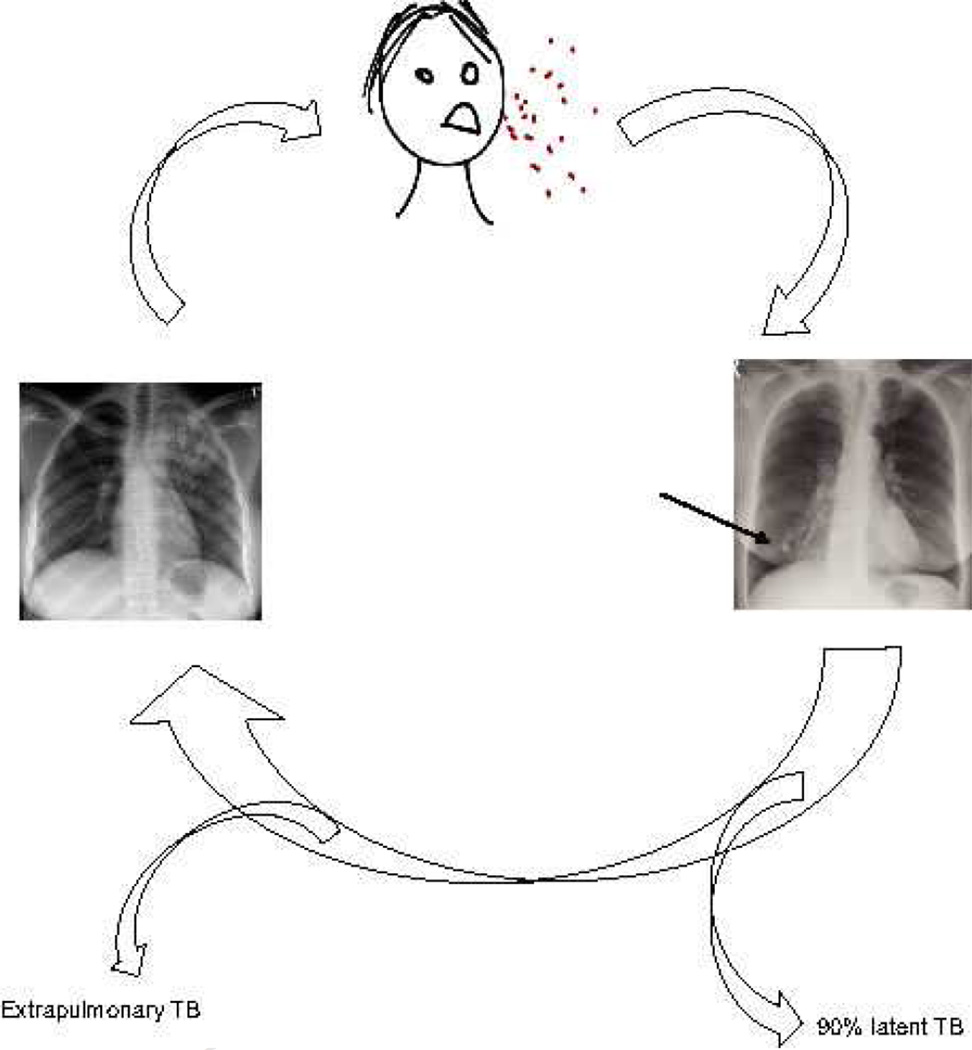
The cycle of TB infection starts with a patient with pulmonary TB coughing, aerosolising bacilli (Figure 1). These infectious droplets are inhaled by close contacts and penetrate the well ventilated lower part of the lungs. The host immune response attempts to control infection, resulting in granuloma formation (4). This initial granuloma may heal, leaving a calcified Ghon focus in the lower zones of the lungs, demonstrating this is the site of initial infection (5). In approximately 90% of exposed patients, Mtb is subsequently controlled and active disease never develops (6). However, in a proportion of patients active TB develops at some point after infection. This may be within months, often as miliary TB, or up to decades later, typically as apical pulmonary TB. The exact perturbation of the immune system which leads to TB reactivation is uncertain. In some cases the immune deficiency is apparent, such as HIV co-infection, alcoholism or treatment with anti-TNF antibodies (4), but in the majority it is unclear.
Figure 1. The life cycle of Mycobacterium tuberculosis.
Bacilli are aerosolised by a patient with pulmonary TB coughing. Droplets are inhaled into the lungs of uninfected patients, where the bacilli multiply and the host immune response attempts to contain infection by the development of a granuloma. A Ghon focus may form at site of implantation (arrow). In about 90% patients, infection is contained for life, but in a subset of those infected, disease reactivates. Only patients with pulmonary disease, and in particular cavitary pulmonary disease, become infectious and re-initiate the cycle. Consequently, each patient must infect over 10 patients to maintain infection prevalence.
Only patients with pulmonary disease transmit infection to new hosts, with the very rare exception of laryngeal disease. Other clinical manifestations, such as lymph node TB, TB meningitis, spinal TB or TB of any other organ, are a biological dead end for the bacilli as they may cause death of the host without onward transmission. Pulmonary TB develops in the apex the lungs (5) and therefore bacilli must spread from their initial seeding point in the lung bases to the apex. The translocation mechanism is uncertain, but the zebrafish model of M. marinum suggests mycobacteria disseminate within infected monocytes (7).
Once lodged at the lung apex, Mtb must subvert the host immune response to drive lung matrix destruction and cavitation, because cavitation leads to very high infectivity (8). The mechanism is not well understood, though is driven by host immunity as cavitation is suppressed in advanced HIV infection (9). Matrix metalloproteinases are emerging as key proteases driving lung matrix destruciton (10, 11). Within the walls of pulmonary cavities, the immune response essentially fails (12) and cavities can contain up to one hundred million bacilli (13). In the pre-antibiotic era, patients were sometime symptomatic for several decades, having “fluctuating consumption”, reflecting the symbiosis that can evolve between the host and microbe when cavities with dense fibrotic rims develop (14). This symbiosis is ideal for Mtb, as it permits a prolonged period of infectivity and thereby allows it to spread to a large number of people.
Therefore, to interrupt this life cycle, improved vaccination strategies are needed to prevent either initial host infection or inhibit Mtb reactivation in the lung apices, or alternatively better diagnostics are required to identify and treat patients with pulmonary disease. As less than 1 in 10 patients who inhale Mtb will ultimately go on to develop pulmonary disease and therefore transmit infection to new hosts, each infectious patient must themselves infect over 10 individuals to maintain the population prevalence (Figure 1). Mtb is surprisingly difficult to transmit, typically only infecting household contacts, and so this number-to-infect ratio represents a potential vulnerability of this highly successful pathogen.
TB VACCINATION
BCG is one of most widely given vaccines and protects children from miliary TB and TB meningitis, and so has been a highly successful vaccine by reducing childhood TB mortality (15). However, BCG does not protect against adult pulmonary disease and so ironically helps the global success of Mtb. BCG protects children from fatal disease that would not be transmitted, but does not prevent adult infectious pulmonary disease. Any new TB vaccination strategy needs to ensure that it increases protection without concurrently worsening adult immunopathology that drives lung cavitation and spread, as alternatively a vaccine may ultimately increase the transmission of Mtb.
TB vaccination represents a significant challenge as even having active pulmonary tuberculosis does not protect from recurrent disease, and in fact may increase the risk of infection (16), so any vaccine strategy must out-perform natural infection. Current vaccines focus on the cytokine-induced T cell response, and they do not consider the effect on extracellular matrix remodelling (15). Therefore, it is unknown whether novel vaccine approaches will increase or reduce the chances pulmonary cavitation and thence transmission. Assessment of vaccine efficacy in the mouse may be misleading as it does not express an orthologue of human matrix metalloproteinase-1 (17, 18), the dominant collagenase driving lung destruction in humans (10). In humans, assessment of a childhood vaccination will require a 25 year follow-up to determine if it protects from adult disease.
One way to circumvent this chronological hurdle is to perform therapeutic vaccination of latently infected adults and determine whether this reduces the incidence of tuberculosis reactivation. If a post-exposure vaccination were to realign the host immune response to a non-tissue destructive phenotype, it would prevent cavity formation and Mtb transmission, and therefore break the cycle of infection. A strategy based on vaccination is much more readily delivered from a policy and implementation perspective, but give the inherent challenges of vaccine development, other points where the infectious cycle can be interrupted should be considered.
IMPROVED DIAGNOSIS OF INFECTIOUS PATIENTS
An alternative strategy is to increase the rate of diagnosis of patients with pulmonary tuberculosis. Each infectious patient must transmit Mtb to over 10 uninfected individuals and therefore this may be a point to suppress the cycle of transmission. TB diagnosis has remained essentially unchanged in many parts of the world for 100 years, with the mainstay of being sputum smear microscopy, but this has relatively poor sensitivity (19). The ideal TB screening test presents an exacting list of requirements, needing to be cheap, robust, require minimal user training, no infrastructure, reagents or electricity and provide results within a few hours (20). The search for a single biomarker of active TB to facilitate such a test has to date been disappointing (21). The development of a test to identify all clinical manifestations of TB, including in patients co-infected with HIV, is difficult, but it may be more straightforward to identify patients with the most pronounced form of disease, pulmonary cavitary TB.
Several novel TB diagnostic assays have been introduced recently. Assays based on interferon gamma release identify whether patients have been exposed to Mtb (22), but do not distinguish latent from active disease and so are not suitable for screening for infectivity. PCR-based diagnostics, such as the Genexpert (23), represent a major advance to diagnose TB rapidly but the cost is not suitable for screening entire populations. Near-patient PCR assays are under development (24) and if the cost can be reduced, these may be applicable to community screening. Analysis of circulating cytokines and chemokines has not identified a panel of markers that is sufficiently specific to TB infection (21), and the analysis of gene transcript signatures is currently only applicable as a research tool (25).
An alternative approach to analysing Mtb components or host immunological parameters is to analyse lung matrix degradation products (MDPs). MDPs are released during both synthesis and degradation of lung matrix, and this remodelling is a central feature pulmonary TB. Collagen turnover releases fragments such as amino-terminal propeptide of type III procollagen (PIIINP), and these are elevated in other destructive pulmonary pathologies (26). Some matrix turnover products, such as desmosine, are really excreted (27) and so matrix turnover products have the potential to be developed into a urine dipstick-based screening tool. Ultimately, an assay incorporating identification of both mycobacterial, immunological and matrix turnover analytes is likely to provide the optimal sensitivity and specificity to identify patients with pulmonary TB within communities. Developing such a test will require collaborative integration of diverse diagnostic approaches.
CONCLUSION
Mtb has continued to spread without interruption for too long, with ongoing transmission preventing TB control in high-burden settings (28). Perhaps now is time to re-focus on the old strategy of actively finding and treating the aerosol supershedders who drive the pandemic in order to break the infectious cycle. The optimal biomarkers for identifying patients with smear positive pulmonary disease need to be identified, and then developed into an affordable near-patient test for population screening.
ACKNOWLEDGEMENTS
PE is grateful for the support of a Higher Education Funding Council for England Clinical Senior Lecturer Award, the US National Institute for Health (R21 AI102239) and a Howard Hughes Medical Institute K-RITH travelling scholar award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328(5980):856–861. doi: 10.1126/science.1185449. Epub 2010/05/15. [DOI] [PubMed] [Google Scholar]
- 2.Raviglione MC, Smith IM. XDR tuberculosis--implications for global public health. N Engl J Med. 2007;356(7):656–659. doi: 10.1056/NEJMp068273. [DOI] [PubMed] [Google Scholar]
- 3.Udwadia ZF. MDR, XDR, TDR tuberculosis: ominous progression. Thorax. 2012;67(4):286–288. doi: 10.1136/thoraxjnl-2012-201663. Epub 2012/03/20. [DOI] [PubMed] [Google Scholar]
- 4.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. Epub 2009/03/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray JF. Bill Dock and the location of pulmonary tuberculosis: how bed rest might have helped consumption. Am J Respir Crit Care Med. 2003;168(9):1029–1033. doi: 10.1164/rccm.200307-1016OE. Epub 2003/09/13. [DOI] [PubMed] [Google Scholar]
- 6.Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362(9387):887–899. doi: 10.1016/S0140-6736(03)14333-4. [DOI] [PubMed] [Google Scholar]
- 7.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136(1):37–49. doi: 10.1016/j.cell.2008.11.014. Epub 2009/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palaci M, Dietze R, Hadad DJ, Ribeiro FK, Peres RL, Vinhas SA, et al. Cavitary disease and quantitative sputum bacillary load in cases of pulmonary tuberculosis. J Clin Microbiol. 2007;45(12):4064–4066. doi: 10.1128/JCM.01780-07. Epub 2007/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwan CK, Ernst JD. HIV and Tuberculosis: a Deadly Human Syndemic. Clin Microbiol Rev. 2011;24(2):351–376. doi: 10.1128/CMR.00042-10. Epub 2011/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, Walker NF, et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest. 2011;121(5):1827–1833. doi: 10.1172/JCI45666. Epub 2011/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker NF, Clark SO, Oni T, Andreu N, Tezera L, Singh S, et al. Doxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinases. Am J Respir Crit Care Med. 2012;185(9):989–997. doi: 10.1164/rccm.201110-1769OC. Epub 2012/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan G, Post FA, Moreira AL, Wainwright H, Kreiswirth BN, Tanverdi M, et al. Mycobacterium tuberculosis Growth at the Cavity Surface: a Microenvironment with Failed Immunity. Infect Immun. 2003;71(12):7099–7108. doi: 10.1128/IAI.71.12.7099-7108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dharmadhikari AS, Nardell EA. What Animal Models Teach Humans about Tuberculosis. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2008-0154TR. Epub 2008/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubos R, Dubos J. The white plague : tuberculosis, man, and society. New Brunswick; London: Rutgers University Press; 1987. [Google Scholar]
- 15.Kaufmann SH, Hussey G, Lambert PH. New vaccines for tuberculosis. Lancet. 2010;375(9731):2110–2119. doi: 10.1016/S0140-6736(10)60393-5. Epub 2010/05/22. [DOI] [PubMed] [Google Scholar]
- 16.Verver S, Warren RM, Beyers N, Richardson M, van der Spuy GD, Borgdorff MW, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med. 2005;171(12):1430–1435. doi: 10.1164/rccm.200409-1200OC. [DOI] [PubMed] [Google Scholar]
- 17.Balbin M, Fueyo A, Knauper V, Lopez JM, Alvarez J, Sanchez LM, et al. Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J Biol Chem. 2001;276(13):10253–10262. doi: 10.1074/jbc.M009586200. [DOI] [PubMed] [Google Scholar]
- 18.Nuttall RK, Sampieri CL, Pennington CJ, Gill SE, Schultz GA, Edwards DR. Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett. 2004;563(1–3):129–134. doi: 10.1016/S0014-5793(04)00281-9. Epub 2004/04/06. [DOI] [PubMed] [Google Scholar]
- 19.Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, Cunningham J, et al. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6(10):664–674. doi: 10.1016/S1473-3099(06)70602-8. Epub 2006/09/30. [DOI] [PubMed] [Google Scholar]
- 20.McNerney R, Daley P. Towards a point-of-care test for active tuberculosis: obstacles and opportunities. Nat Rev Microbiol. 2011;9(3):204–213. doi: 10.1038/nrmicro2521. Epub 2011/02/18. [DOI] [PubMed] [Google Scholar]
- 21.Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol. 2011;11(5):343–354. doi: 10.1038/nri2960. Epub 2011/04/09. [DOI] [PubMed] [Google Scholar]
- 22.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4(12):761–776. doi: 10.1016/S1473-3099(04)01206-X. Epub 2004/11/30. [DOI] [PubMed] [Google Scholar]
- 23.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005–1015. doi: 10.1056/NEJMoa0907847. Epub 2010/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikam C, Jagannath M, Narayanan MM, Ramanabhiraman V, Kazi M, Shetty A, et al. Rapid Diagnosis of Mycobacterium tuberculosis with Truenat MTB: A Near-Care Approach. PLoS ONE. 2013;8(1):e51121. doi: 10.1371/journal.pone.0051121. Epub 2013/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466(7309):973–977. doi: 10.1038/nature09247. Epub 2010/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lammi L, Ryhanen L, Lakari E, Risteli J, Paakko P, Kahlos K, et al. Type III and type I procollagen markers in fibrosing alveolitis. Am J Respir Crit Care Med. 1999;159(3):818–823. doi: 10.1164/ajrccm.159.3.9805060. Epub 1999/03/02. [DOI] [PubMed] [Google Scholar]
- 27.Turino GM, Ma S, Lin YY, Cantor JO, Luisetti M. Matrix elastin: a promising biomarker for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184(6):637–641. doi: 10.1164/rccm.201103-0450PP. Epub 2011/07/16. [DOI] [PubMed] [Google Scholar]
- 28.Nardell E, Churchyard G. What is thwarting tuberculosis prevention in high-burden settings? N Engl J Med. 2011;365(1):79–81. doi: 10.1056/NEJMe1105555. Epub 2011/07/08. [DOI] [PubMed] [Google Scholar]