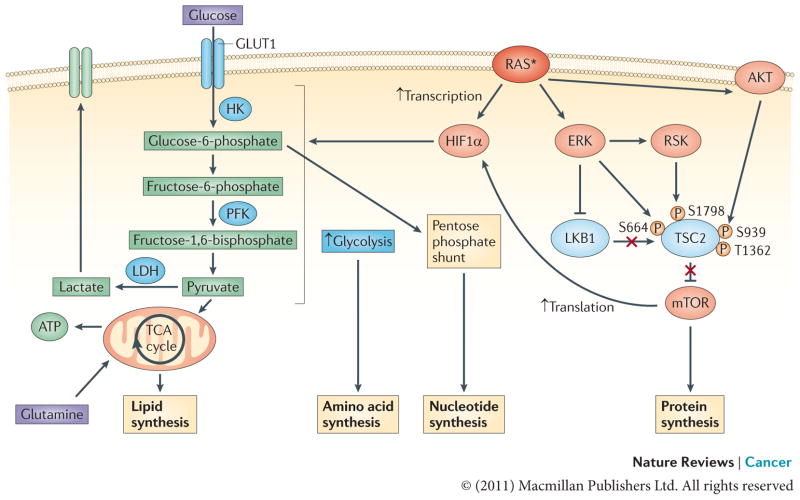
Figure 4. Effect of RAS on energy metabolism in cancer cells: generating macromolecular precursors.
ERK and PI3K signalling downstream of oncogenic RAS converge to activate mTOR by inhibiting its negative regulators tuberin (TSC2) and liver kinase B1 (LKB1)–AMP-activated protein kinase (AMPK)116. TSC2 can be directly phosphorylated by both ERK and ERK-activated ribosomal protein S6 kinase (RSK) on S664 and S1798, respectively, as well as by AKT (on S939 and T1362), and, likewise, RAF–ERK1 or RAF–ERK2 signalling disrupts the LKB1–AMPK checkpoint200–204. This leads to mTOR–eukaryotic translation initiation factor 4 (eIF4)-dependent translation of hypoxia-inducible factor 1α (HIF1α). Activated RAS can also result in the transcriptional upregulation of HIF1A. Increased levels of HIF1α augment multiple steps in glycolytic metabolism (shown in blue). The upregulation of hexokinase (HK) facilitates the conversion of glucose to glucose-6-phosphate, a glycolytic intermediate that is used in pentose phosphate pathway-dependent nucleotide synthesis205. Higher levels of phosphofructokinase (PFK) lead to an enhanced glycolytic flux and the production of pyruvate, which, in conjunction with the oncogenic RAS-dependent increase in lactose dehydrogenase (LDH) levels, can allow glycolysis to persist by regenerating NAD+, a necessary cofactor for glycolytic reactions109,120,121. In addition, some of the pyruvate can enter the tricarboxylic acid (TCA) cycle where its conversion to citrate generates intermediates that are necessary for the synthesis of fatty acids and non-essential amino acids205. The asterisk represents the mutational activation of RAS. GLUT1, glucose transporter 1.