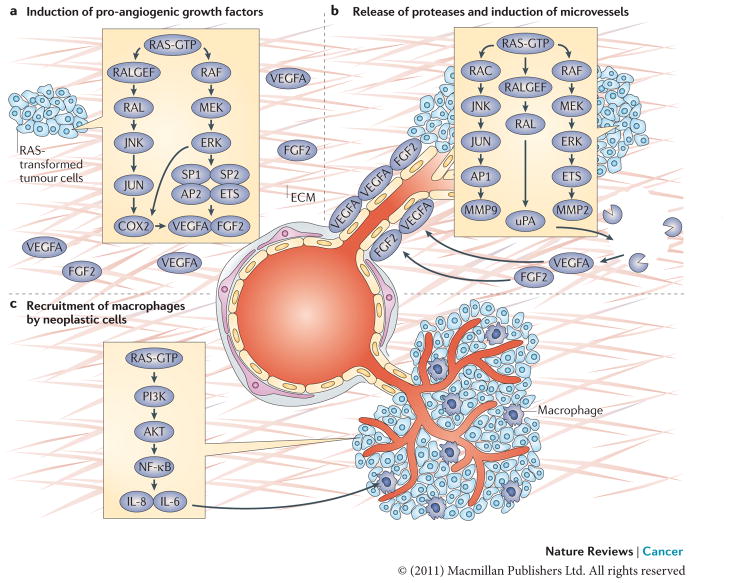
Figure 5. RAS and angiogenesis.
a. The induction of pro-angiogenic growth factors (vascular endothelial growth factor A (VEGFA) and fibroblast growth factor 2 (FGF2)) by RAS in neoplastic cells is shown. RAS enhances the transcription of VEGFA by recruiting transcription factors such as SP1, SP2, AP2 and ETS to the VEGFA promoter. RAS also increases the stability of VEGFA mRNA and augments its translation206–213. The RAS–JUN N-terminal kinase (JNK) signalling axis is responsible for upregulating the transcription of prostaglandin-endoperoxide synthase 2 (PTGS2), which encodes COX2, by activating JUN, a component of the AP1 transcription complex, whereas the RAS–ERK1 and ERK2 pathway contributes to COX2 expression through the phosphorylation of CCAAT/enhancer binding protein-β (C/EBPβ) and ETS transcription factors such as PEA3 (REFS 214–217). Expression of COX2, in turn, increases the levels of VEGFA produced by RAS-transformed cells. b. The release of proteases by neoplastic cells cleaves components of the extracellular matrix (ECM) and releases VEGFA and FGF2, which are trapped in the ECM. Expression of proteases urokinase-type plasminogen activator (uPA), matrix metalloproteinase 2 (MMP2) and MMP9 in RAS-transformed cells is increased by the combined effects of ETS transcription factors (activated by the RAF–ERK pathway) and JUN (activated by the RAC–JNK pathway) binding to the promoters of PEA3–AP1 sites, as well as enhanced translation of polysome-associated MMP9 mRNA144,218,219. Stimulation of uPA expression is also dependent on RAS-mediated activation of RAL GTPase220,221. This induces neo-proliferation and sprouting of microvessels towards the tumour site. c. The recruitment of macrophages by neoplastic cells (through RAS-induced nuclear factor-κB (NF-κB)-dependent production of the cytokines interleukin-6 (IL-6) and IL-8) and subsequent promotion of endothelial proliferation and sprouting by newly recruited macrophages is shown.