Abstract
The tumor-associated stroma has been described in recent years as being complicit in tumor growth in pancreatic cancer. The stroma hosts a variety of components of both cellular and molecular makeup. In normal tissues, the stroma provides nutrients and regulatory signals for proper cellular polarity and function. However, following oncogenic transformation, the stromal compartment is conscripted to provide stimulatory signals and protection to tumor cells. It is these tumor–stromal interactions that are currently of great therapeutic interest. Several key reports have suggested that therapeutic targeting of the tumor–stromal interactions in pancreatic cancer has the potential to offer survival benefit. In this review, we will discuss the tumor–stromal interactions that contribute to tumor growth and progression, and ways in which we might counter these interactions.
Keywords: tumor microenvironment, extracellular matrix, pancreatic cancer
I. INTRODUCTION
Stephen Paget put forth his ‘seed’ and ‘soil’ hypothesis in 1889, suggesting for the first time that the incidence of cancer metastasis to a specific organ site did not occur in a stochastic fashion but rather with remarkable prejudice.1 Studies of the interaction of the tumor cell, or ‘seed,’ with the adjacent and distal tissues, or ‘soil,’ he suggested, would provide a better understanding of tumor cell growth. Historically, in the context of the tissue, the tumor-associated ‘soil’ or stroma (cellular and extracellular tissue component not necessary to the primary tissue function) has been described relative to the parenchyma (primary cell type recognized as vital to proper tissue function, e.g., epithelial cell) and has been ignored in its possible complicit relationship to tumor growth. In more recent years, we have come to a greater understanding of the composition and nature of the stroma, that it includes fibroblasts, stellate cell derived myofibroblast-like cells, and, to varying degrees, an assortment of immune cell types, each of which contributes to the growth and survival of tumor cells. Moreover, we know now that each of these cell types plays important roles in supporting and even enhancing tumor growth and survival (Fig. 1). This is particularly true in pancreatic cancer.
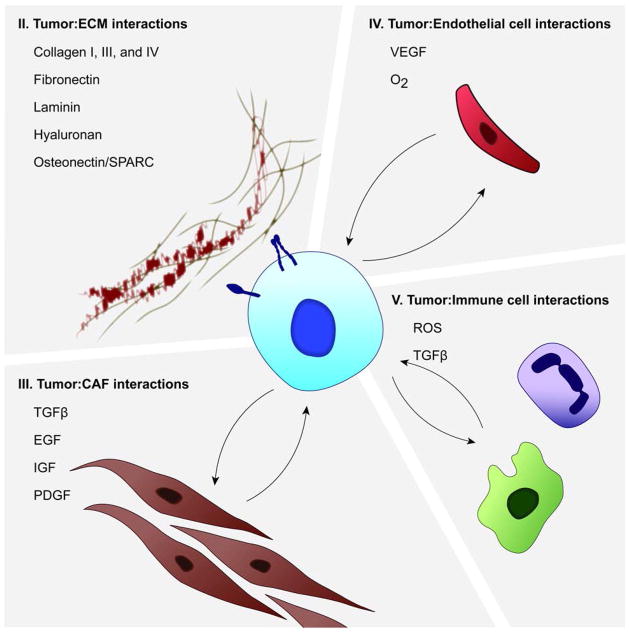
FIGURE 1.
Tumor-stroma interactions in pancreatic cancer. PDAC is characterized by the development of the desmoplastic reaction, which features growth of the CAF cell compartment, and deposition of ECM components. Reciprocal growth factor signaling results in increased expression of growth factors such as IGF, EGF, and TGFβ. In addition, interactions with the immune cell and endothelial cell compartment contribute to tumorigenesis through the release of ROS and additional growth factors. Interactions with each of these compartments contribute to the enhanced growth of tumors in PDAC. Each quadrant heading corresponds to a section title and topic to be covered in the following review.
Pancreatic ductal adenocarcinoma (PDAC) currently has the lowest five-year relative survival rate of all cancer types.2 As symptoms can be vague, and as the pancreas is inaccessible to routine examination, many patients present with advanced, metastatic disease at diagnosis. Many factors combine in PDAC to make it refractory to common therapies, and inoperable in all but a small fraction of those diagnosed. PDAC has a high propensity for metastasis, even more significantly limiting the outlook of patients often diagnosed at a late stage of disease. PDAC is characterized by extensive fibrosis in what is termed the desmoplastic reaction (Fig. 2).3 This desmoplastic reaction arises primarily from activated myofibroblast-like cells that secrete large quantities of extracellular matrix proteins, including collagen types I, III, and IV, into the tumor microenvironment.4 The secretion of extracellular matrix proteins contributes to the disease phenotype seen in PDAC, especially as collagens have been shown to be important in enhancing tumor growth by sequestering multiple growth factors and decreasing anti-cancer drug penetration (see Table 1 for a description of components). The highly fibrotic stromal component of PDAC, including abundant collagen and hyaluronan, also likely contributes to diminished clinical efficacy of anti-cancer agents.5–7 Pancreatic cancer, therefore, presents several unique challenges, including poor vascularity resulting in poor perfusion of the tumor mass, despite the increased expression of proangiogenic factors.8,9
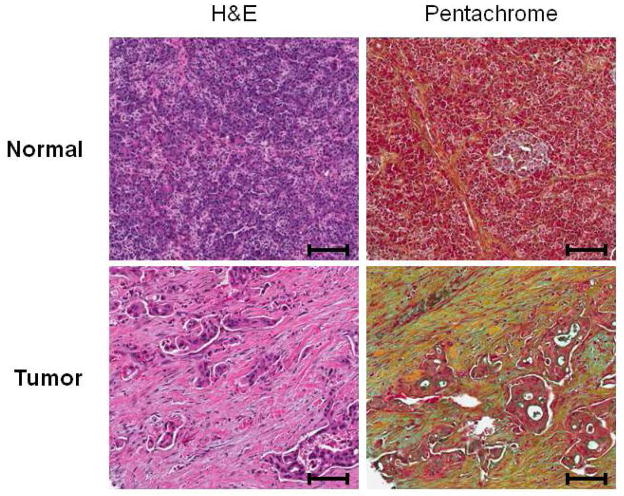
FIGURE 2.
Histochemical analysis of human pancreatic tissue samples. Both normal and tumor pancreatic tissues were subjected to hematoxylin/eosin (H&E) and pentachrome (Russell-Movat’s) staining analysis. H&E analysis reveals increased proliferation of the pancreatic stellate cell population in the tumor tissues relative to normal tissues. Pentachrome analysis also demonstrates increased collagen expression in the stromal compartment in tumor tissue relative to normal tissue. Pentachrome staining: Green/blue=mucins, Yellow=collagen, Red=muscle/fibrinoid. Scale bar=100 μm
TABLE 1.
Components of the stromal compartment. The stromal compartment is comprised of many components, both cellular and molecular in makeup. Each of these components is thought to contribute to cancer tumorigenesis through multiple mechanisms.5, 54, 109–116 Targeting the stromal compartment in pancreatic cancer may offer therapeutic benefit by enhancing the efficacy of current treatment regimens.
| Type | Component | Role in tumorigenesis | Reference |
|---|---|---|---|
| Cellular | |||
| Cancer-associated fibroblast | Provide survival signals and secrete ECM | 115 | |
| Endothelial cell | Recruit inflammatory immune cells | 116 | |
| Infiltrating immune cell | Provide survival signals, promote instability | 79 | |
| Proteinaceous | |||
| Collagen I, III, IV | Promotes proliferation and inhibits perfusion | 54 | |
| Decorin | Harbors TGFβ ligand | 5 | |
| Versican | Promotes proliferation, and apoptotic resistance | 114 | |
| Fibronectin | Promotes apoptotic resistance | 112 | |
| Laminin | Promotes apoptotic resistance | 113 | |
| Osteonectin/SPARC | Promotes proliferation | 16 | |
| Heparin sulfates | Promotes tumor progression | 109 | |
| Keratin sulfates | Poor survival if expressed in stroma | 110 | |
| Non-proteinaceous | |||
| Hyaluronan | Promotes proliferation, survival, and invasion | 111 | |
With these and other observations, it has become clear that targeting the tumor-stromal interaction may be an important therapeutic component in enhancing current and future therapies that target tumor cell-specific functions or pathways. While a few approaches have been used to target the tumor microenvironment, as will be seen below, these studies have been met with failure in a few initial studies. In this review, we will discuss a few key tumor-stromal interactions that function in enhancing tumor growth and progression of disease. In addition, we will outline some specific findings, including discussion of unique pathways and targets in the literature that may guide future endeavors in the treatment of patients with pancreatic cancer.
II. TUMOR–ECM INTERACTIONS
Normal cellular interactions with the extracellular matrix (ECM) play an important role in the control of multiple cellular processes, including cell polarity, proliferation, and migration. Tight control over polarity and cellular proliferation of the epithelial compartment is important to normal glandular function in the pancreas, and in preventing dysplastic growth. Where this interaction is severed; an anchorage-dependent cell death program (or ‘anoikis’) is initiated to prevent metastatic displacement and growth.10,11 Normal cellular interactions with the ECM are maintained through the ligand binding of the various ECM proteins with the extracellular domain of the integrin family of receptors. On the other hand, interactions between cells and certain ECM glycosaminoglycans, namely hyaluronan, are maintained by ligand binding with the CD44 or hyaluronan-mediated motility (RHAMM) receptors. Frequently in cancer, however, ECM receptor expression is dysregulated. While expression of the various receptors is largely tissue and cell-type specific, integrin expression in cancer, for example, generally favors greater expression of those integrin receptor subtypes that allow for anchorage-independent growth and migration, while disfavoring expression of the receptor subtypes that would promote adhesion or cell death. By means of receptor subtype expression switching, cells are able to exert greater control over their survival pathways.
A. Integrin Signaling
Integrin signaling in pancreatic cancer promotes survival via multiple mechanisms. In human cells, integrin signaling is mediated by the expression of eighteen α and eight β subunits, which can interact in a variety of combinations to form as many as twenty four unique receptors with differing affinities for the ECM proteins.12 In pancreatic cancer, multiple integrin subunits have been shown to be dysregulated. With as many as six α and one β subunit showing increased expression, and one α and two β showing decreased expression, the expression trends are not altogether clear.13,14 However, some of the integrin receptor combinations reported to promote cell survival generally in cancer include: αvβ3, αvβ6, and α6β4.10 Indeed, α6β4 ligation has been shown to promote tumorigenesis, including enhancing the expression of the extracellular glycoprotein, secreted protein acidic and rich in cysteine (SPARC), by suppressing the expression of miR-29a and enhancing cell invasion.15 Stromal SPARC expression correlates with poor prognosis in pancreatic cancer and may serve as a suitable prognostic marker for both survival and treatment outcomes.16,17
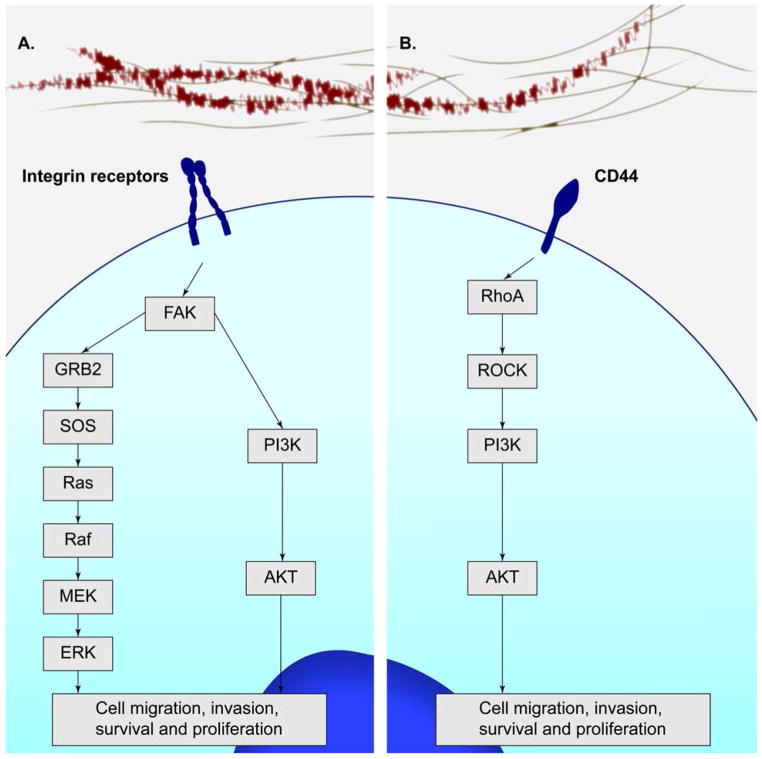
Normal integrin signaling functions directly via focal adhesion kinase (FAK) and either GRB2/SOS/Ras, or via the PI3K-AKT signaling axis (Fig. 3). The integrin family of receptors promotes cell survival in cancer by, among others, increasing the Bcl-2/Bax ratio, inactivating p53, or by activation of the NF-κB or PI3K-AKT pathways.18–23 It has been shown that α5β1 integrin expression, for example, induces increased expression of Bcl-2 in a fibronectin-dependent manner, without increased expression of Bax.24 Here, it appears that the α5 subunit is required for survival as isogenic cells expressing αvβ1 do not survive serum withdrawal unless exogenous Bcl-2 is provided. Also, β1 integrin binding and the consequent increases in Bcl-2 expression function to increase proteasomal degradation of the pro-apoptotic Bim protein, thus increasing cellular resistance to anoikis.25,26 These mechanisms ultimately set the stage to allow for metastatic growth where cell anchorage does not inhibit the proliferation of the epithelial cell. Integrin signaling, however, is not the only means of cellular communication with the ECM.
FIGURE 3.
Primary signaling pathways mediated by the integrin receptors and CD44. Integrin signaling functions directly via FAK and either GRB2/SOS/Ras, or via the PI3K-AKT signaling axis. The integrin family of receptors promotes cell survival, proliferation, migration, and invasion. CD44 also mediates PI3K-AKT signaling via RhoA/ROCK, which promotes cell survival, proliferation, and migration.
B. CD44 Signaling
The deposition of hyaluronan (HA), an extracellular matrix glycosaminoglycan, is also increased in pancreatic tumor tissues.27 The binding of HA to CD44 has been shown to increase cell survival. CD44 is the transmembrane receptor for hyaluronan and is encoded by a single gene. Multiple forms of the receptor, however, are expressed by alternative splicing mechanisms, and by post-translational modifications, including N- and O-linked glycosylation.28 Cancer cells often express one of the variant CD44v isoforms (v1–v10). Similar to the integrins, CD44 functions to control the positional localization of receptor tyrosine kinases by managing their temporal clustering. It has been reported that subsequent to CD44-induced clustering, matrix metalloproteinase 9 (MMP9) trapping and TGFβ activation can occur, leading to ECM remodeling and greater oncogenic potential.28,29 Furthermore, it has been suggested that HA:CD44v6 interaction mediates a positive feedback loop wherein PI3K signaling is maintained resulting in greater tumor cell invasiveness.28,30 These results are supported by additional observations showing that CD44 may enhance survival and proliferation signals in a HA-dependent manner via a TGFβR/EGFR-mediated signaling mechanism.31 These examples suggest that, like the integrin signaling, CD44 is capable of signaling through pathways commonly exploited in cancer cells to circumvent anti-proliferative restraint. In pancreatic cancer, where hyaluronan can be found in markedly increased concentrations, the HA:CD44 interaction may employ these pathways to enhance cell survival and growth. Indeed, recent reports have shown that a pegylated form of a humanized HA degrading enzyme, hyaluronidase, was effective at significantly enhancing gemcitabine activity and overall survival in a transgenic mouse model for PDAC.32,33
Integrin and CD44 interaction with their respective extracellular matrix counterpart function to enhance survival and proliferation of cancer cells. Deregulation of normal integrin and CD44 signaling is vital to tumor formation. Indeed, abrogation of dysregulated β1-integrin:ECM signaling can induce tumor reversion to a near-normal phenotype in some cancer models, including HMT-3522 breast cancer cells, even in the presence of oncogenic genetic alteration.34 While deregulation of tumor cell–ECM signaling pathways are necessary for resistance to anoikis, it is important to note that the tumor epithelial cell is not the primary source of ECM components in pancreatic cancer. The pancreatic cancer associated fibroblast is the primary source of tumor-associated collagen and hyaluronan synthesis.32,35 While integrin and CD44 signaling mediate the tumor cell–ECM interaction, tumor cells and CAFs interact in a paracrine fashion via several important pathways to further enhance their survival and proliferative potential.
III. TUMOR–CAF INTERACTIONS
Desmoplasia is characterized by significant production of extracellular matrix proteins and extensive proliferation of myofibroblast-like cells.4 These myofibroblast-like cells, also known as activated pancreatic stellate cell or simply cancer-associated fibroblasts (CAF), have been identified as the primary synthetic source of many of the extracellular matrix components, both in hepatocellular and pancreatic carcinoma.4,36 Under normal conditions, the quiescent stellate cell interacts with the epithelial cell compartment by serving as a tissue reservoir for vitamin A. Activation of the stellate cell, however, results in changes to stellate cell morphology, proliferation rate, and sensitivity to mitogenic factors. The activated CAF is known to be a primary source of many mitogenic factors in tumors, including insulin growth factor (IGF), epithelial growth factor (EGF), and transforming growth factor β (TGFβ).37,38 Increased expression of TGFβ has been observed in breast, hepatocellular, lung, and in pancreatic cancer. Furthermore, this increased expression correlates with tumor progression and metastasis as well as poor clinical outcome.39 The role and magnitude of the pleiotropic effect of these growth factors in pancreatic cancer is not fully understood, especially as TGFβ can have both tumor promoting and suppressing effects in some models.40 However, recent research has pointed out that the net effect of TGFβ, for example, in pancreatic cancer is initially tumor suppressive, but becomes tumor promoting as disease progresses.39 Thus, the tumor cell–CAF interaction ultimately supports tumor progression by providing these necessary and important growth factors, including TGFβ.
A. TGFβ Signaling
Members of the TGFβ family of cytokines have been implicated in the invasiveness of tumor cells, as well as in the activation of the CAF that leads to pancreatic fibrosis. Member proteins are multifunctional cytokines known to be involved in a host of cellular functions, including immune cell regulation, growth and differentiation, and in cell migration.38 Currently, three different TGFβ ligands (TGFβ1-3) have been described. TGFβ signaling is mediated following TGFβ ligand binding to one of three types of TGFβ receptors (TGFβR), and activation of downstream Smad signaling. In many cells, this is achieved via TGFβRI (also known as ALK5) signaling in concert with a type II TGFβR. Smad4/DPC4 is reportedly deleted or inactivated in approximately 55% of pancreatic tumors; it may be that the TGFβ-associated correlations between increased tumorigenesis and poor outcome may be the result of Smad-independent pathways that play a critical role in the TGFβ-dependent tumor cell invasiveness in some tumors.41 Indeed, wild-type Smad4/DPC4 corresponds to decreased invasive potential and better prognosis in pancreatic cancer patients.42,43 The Smad-independent pathways include, among many others, RHOA, Ras, PI3K, and MAP3K1.44–47 Some of the early studies that established a role for TGFβ in tumor development employed a tetracycline-inducible MMTV-TGFβ transgenic mouse. Using the oncogenic capability of constitutive MMTV-PyVmT expression, the authors noted as much as a ten-fold greater incidence of metastases to the lung following TGFβ induction.39,48 Despite the complexity and multifunctional nature of the signaling pathways, recent studies have indicated that intervention with TGFβ inhibitors can have therapeutic benefit, without the danger of many of the expected side-effects including enhancement of cell growth.49–51 Interestingly, it was observed that TGFβR1 haploinsufficiency can itself significantly inhibit the development of fibrosis and progression of precancerous lesions in mice, leading to further studies looking closely at the effects of TGFβ inhibition in fibroblast cells.52 Furthermore, due to the important nature of TGFβ in the perpetuation of CAF activation, studies have focused on employing TGFβ antagonists in therapeutic intervention of fibrosis in chronic pancreatitis.53 One report has demonstrated that fibrosis can confer drug resistance in in vitro pancreatic tumor models.54 How exactly this is accomplished has yet to be determined. However, it seems clear that extracellular matrix components can confer resistance in vivo at least in part by decreasing interstitial drug penetration and transport.6,32,33 Some research suggests that resistance may also come about following an epithelial-to-mesenchymal transition (EMT) in the tumor cells that is induced by TGFβ and MMP expression, resulting in the altered expression of multiple genes thought to be involved in decreased drug sensitivity.55 This is true of erlotinib resistance in head and neck squamous cell carcinoma (HNSCC) cells wherein greater resistance to erlotinib corresponds to increased Zeb-1 (also known as deltaEF1) expression, resulting in decreased E-cadherin expression and EMT, which is a direct result of TGFβ ligand binding and Smad nuclear translocation.56,57 Targeting of EMT may show some promise in pancreatic cancer as it appears, for example, that targeting tumor EMT and invasion with the mucin-reactive PAM4 antibody may improve treatment efficacy.58–60 Other approaches to targeting EMT include the Secreted clusterin (sCLU)-reactive monoclonal antibody AB-16B5.61
Tumor–CAF interaction is multifaceted. It involves many growth factors signaling in reciprocal fashion to effect increased cell proliferation. These growth factors also contribute to tumor progression by enhancing the CAF-dependent deposition of ECM proteins or fibrosis. Fibrosis can then mediate tumor progression at both the molecular and tumor tissue level. Each of these features of the tumor microenvironment enhances epithelial cell proliferation and capacity for escaping the epithelial cell compartment. The endothelial cell compartment, however, also contributes to tumor growth.
IV. TUMOR–ENDOTHELIAL CELL INTERACTIONS
Angiogenesis, or the formation of new blood vessels, is a complex process requiring the coordination of multiple cell types and multiple mitogenic factors. Angiogenesis has been recognized for some time to be vital to the growth and progression of primary tumors and metastases.62,63 Following the work of the late Dr. Judah Folkman, intense effort has been put into developing drugs targeting angiogenesis in tumors. With the 2007 approval of the anti-vascular endothelial growth factor-A (VEGF-A) monoclonal antibody therapeutic, bevacizumab (Avastin®), many have touted anti-angiogenic approaches in a variety of cancers.64 Indeed, bevacizumab shows synergistic efficacy in multiple tumor types, including metastatic colorectal cancer, recurrent or metastatic non-squamous non-small cell lung cancer (NSCLC), and in the treatment of metastatic renal cell carcinoma. Certainly, the success of such an approach has validated the notion that targeting some of the stromal components of a tumor can offer clinical benefit. Unfortunately, however, bevacizumab failed to show any significant clinical beneft in treating patients with PDAC.65,66 While terribly disappointing, the failure of bevacizumab in PDAC can offer some insight into additional considerations of pancreatic biology that must also be made in the development and design of drugs for PDAC. First, while pancreatic tumor cells do show increased expression of multiple mitogenic factors, including VEGF-A, the lack of studies validating the role of angiogenesis in PDAC underscores the necessity for further studies in PDAC to understand its role in PDAC.67 One recent study indicated that, in fact, human pancreatic tumors, as well as mouse pancreatic tumors from genetically engineered mice, show significantly reduced vascularity relative to normal pancreatic tissue.68 While, on the surface, this study appears to refute some of the generally accepted notions of angiogenesis in cancer biology, it has uncovered unique characteristics of the PDAC microenvironment and highlights our lack of understanding of tumor vasculature in the specific context of pancreatic cancer.
In their seminal paper on the potential efficacy of hedgehog inhibitors in pancreatic cancer, Olive et al outlined several impressive experiments demonstrating that in fact mouse pancreatic tumors are poorly vascularized, and show decreased drug perfusion relative to transplanted xenograft tumors or normal pancreatic tissue.68 Hedgehog pathway inhibitors as a class, including the inhibitor IPI-926 (Infinity Pharmaceuticals) used by Olive and colleagues, potentially target multiple points in the hedgehog pathway. Sonic hedgehog, Patched, and Smoothened are also known to play important roles in angiogenesis. The angiogenic effects of these pathways have also been observed in ischemic limbs and in the cornea of adult mice, or in rat models.69,70 However, Olive and others have shown that the efficacy of the hedgehog inhibitors in treating PDAC in their model (with an increase in overall survival) occurs through a mechanism that potentiates perfusion.71 Indeed, their results demonstrated one curious aspect of their hedgehog inhibition in that it increased endothelial cell proliferation and decreased CAF proliferation. Unfortunately, however, in January, 2012, a randomized phase II study employing gemcitabine plus IPI-926 versus gemcitabine plus placebo (clinicaltrials.gov, NCT01130142) in pancreatic cancer was halted following an interim review that disclosed that the study would fail to meet its overall survival endpoint in extending survival beyond historical controls.72
Provenzano and colleagues have added another approach to improve perfusion of pancreatic cancer in their genetically engineered mouse model. They noted that dysregulated vascular diffusion and convection could be surmounted via hyaluronidase treatment of tumors, resulting in decreased interstitial pressures (Pi), from around 75–150mm Hg in untreated tumors to 20–30mm Hg in hyaluronidase treated tissues, and that the reduction corresponded to improved survival outcomes.32 With the failure of IPI-926, however, additional studies characterizing the role of hedgehog signaling, hyaluronan, and the ultimately the tumor–endothelial cell interaction in tumor progression will be necessary.
It is clear that angiogenesis plays a unique role in PDAC. The significant desmoplasia and poor vasculature that are so characteristic to PDAC could conspire against most regimens to prevent adequate clinical efficacy. Certainly, regimens including therapeutics designed to increase drug perfusion or improve tumor vasculature, however this is accomplished, should be considered.
V. TUMOR–IMMUNE CELL INTERACTIONS
In 1863, Rudolf Virchow observed that leukocytes could infiltrate into tumors, suggesting a potential association between inflammation and cancer.73 From his observations, he suggested that chronic inflammation may play a role in contributing to the development of cancer. As the presence of leukocytes in tumor infiltrates alone could not enable one to discern the immune system’s role in cancer, whether in perpetuating or destroying a tumor, significant effort has been made in characterizing the immune response to tumor cells, or other conditions that may initiate tumorigenesis. It is clear from modern studies that many infectious agents are associated with the development of malignancies, such as is the case with schistosomiasis and bladder cancer, Helicobacter pylori and stomach cancer, or human papillomavirus and cervical cancer.74–76 However, chronic and unregulated inflammation is also thought to play a causative role as well. Indeed, hereditary pancreatitis is a known etiologic factor for PDAC, resulting in a lifelong cumulative rate of pancreatic cancer risk as high as 40% in those untreated.77 Unfortunately, as is the case in many cancers, the inflammatory response can lead to a state that may promote tumors by increasing genomic instability and secretion of growth factors.78 For example, while neutrophil-mediated superoxide respiratory burst is necessary for destroying pathogenic organisms, the resulting reactive oxygen species (ROS) are thought to contribute significantly in increasing genomic instability at sites of chronic inflammation.79 The ROS generated by neutrophils and macrophages can contribute to genotoxicity, including DNA strand breaks or base modifications. The ROS generated by neutrophils and macrophages can also result in the activation of potent carcinogens.79,80 However, strong evidence demonstrating a link between immune system response and pancreatic cancer has come in a report demonstrating that conditional mutant KRAS expression and p16Ink4a/p19Arf or p53 loss was insufficient for oncogenic transformation in pancreatic acinar cells in mice.81 Rather, it was following cerulein-induced pancreatic inflammation and pancreatitis, which involves inflammatory cell infiltration, that transformation occurred. This and other studies demonstrate a direct link between immune system responses and progression in cancer. So, it is no surprise that immune cells also probably play a central role in the development of pancreatic cancer. As the immune system’s inflammatory response is a coordinated effort requiring participation among many different components, multiple cell types are known to take part in this complex process in the human disease. Indeed, the tumor-associated macrophages (TAM), neutrophils, and regulatory T cells (Treg) are among the many inflammatory cell types that infiltrate tumors and play an important role in the tumor stroma.82,83
A. Tumor-Associated Macrophages
TAMs are among the most abundant immune cell type to extravasate into the tumor microenvironment. TAMs are recruited to tumor tissues by the CC chemokines CCL2, 3, 4, 5, and 8. TAMs can also be recruited via stromal-derived factor 1 (SDF-1), VEGF, colony stimulating factor 1 (CSF-1), and thrombospondin-1 (TSP-1).84–86 Once activated, macrophages secrete various growth factors and cytokines into the surrounding tumor tissue, contributing greatly to an altered stromal environment supportive of tumor growth. TAMs themselves are known to express a variety of pro-angiogenic factors, including VEGF-A, urokinase plasminogen activator (uPA), and thymidine phosphorylase (TP), which can each contribute to enhanced tumor growth.87, 89 In addition to proangiogenic factors, TAMs are also known to secrete lymphangiogenic factors, including VEGF-C and VEGF-D. The functional development of the lymph system in neoplastic tissues creates avenues by which tumors can more readily metastasize.85 TAMs also function within the tumor microenvironment in an immunosuppressive capacity. TAMs are known to express and secrete IL-10. IL-10 has been shown to inhibit the differentiation of monocytes to dendritic cells (DC), and thus prevent the DC-mediated anti-tumor response.90 Finally, macrophages have been shown to stimulate collagen type I and C-fibronectin synthesis via a TGFβ-dependent mechanism mediated by the CAF.91 This finding, in particular, demonstrates that the infiltration of leukocytes to the stromal compartment can vastly alter its properties and hijack it to support tumor growth.
B. Neutrophils
Neutrophils also play an important role within the tumor stroma. They function in the acute inflammatory response, releasing toxic granules that are a significant source of ROS. Neutrophils are also a source of additional tumor-supporting molecules. One report by Nozawa et al. suggests that neutrophils mediate the angiogenic switch via MMP-9 expression.92 In their report, they demonstrated that neutrophil depletion resulted in significantly decreased VEGF–VEGF receptor association further demonstrating a significant role in angiogenic development. Other studies have shown that neutrophils can also enhance the propensity for metastases in certain models. In their report, Tazawa et al. demonstrated that neutrophil infiltration correlated with an increase in metastatic events.93
C. Regulatory T Cells
Finally, it is also clear that regulatory T cells play an important role in the immune response to cancer. While their role in the immune response may not induce carcinogenesis or mutagenesis, the regulatory T cell functions to suppress the response of the immune system toward the cancer cells. Indeed, reports have demonstrated that the prevalence of Treg cells is increased in those with either PDAC or breast adenocarcinoma, suggesting that the Treg cell may play a significant role in suppressing an immune response in PDAC.94,95 The Treg cell coexpresses CD4 and CD25, as well as the transcription factor FOXP3.96 It functions within the tumor microenvironment by expressing IL-10, TGFβ, and cytotoxic T-lymphocyte antigen 4 (CTLA4), all of which have been shown to exhibit anti-inflammatory effects and to participate in suppressing auto-immunity.97–100 Potentiating the immune system response to pancreatic cancer has been investigated with the anti-CTLA4 therapeutic, ipilimumab (Yervoy®). While it was reported to be ineffective as a single agent in a phase II study (clinicaltrials.gov, NCT00112580), some have suggested that immune cell modulation may yet be an effective strategy.101
Several additional approaches targeting the deleterious effects of the immune system in cancer are currently being tested in clinical trials, including the immune cell targeting biological denileukin diftitox (ONTAK®) and anti-Tac (Fv)-PE38 (LMB-2) (clinicaltrials.gov, NCT00726037 and NCT00924170, respectively). Denileukin diftitox is a recombinant fusion protein that combines the binding portion of IL-2 with the catalytic portion of diphtheria toxin (DT). The receptors for IL-2 include CD25, CD122, and CD132, and are known to be highly expressed on Treg cells. The catalytic portion of the DT catalyzes the transfer of the ADP-ribose moiety of nictotinamide adenine dinucleotide (NAD) to the host cells’ elongation factor 2 (EF-2), and functions by inhibiting protein synthesis which results in apoptosis.102 This therapeutic approach has enabled clinicians to selectively deplete tissues of the regulatory T cells, and has been shown to increase the anti-tumor immune response of effector T cells in multiple models.103,104 While denileukin diftitox’s poor efficacy in metastatic melanomas may in part be due to an inability to completely deplete tumors of Treg cells, validation of a Treg cell depletion approach has been demonstrated in several studies.105,106 The anti-Tac(Fv)-PE38 immunotoxin utilizes a similar approach to denileukin diftitox. The anti-Tac (Fv)-PE38 immunotoxin is a recombinant fusion protein which fuses the Fv portion of a monoclonal antibody specific to the IL-2 receptor (CD25) to a Pseudomonas exotoxin.107 In addition to these immunotoxin approaches, others have suggested that blockade of the naïve T cell-to-Treg conversion, rather than outright depletion of Treg cells, would be a more effective approach especially in light of the finding that tumors depleted of regulatory T cells are subsequently replenished following conversion of naïve T cells found in the tumor periphery.108 This approach is still under investigation.
Immune cell interactions between the microenvironment and tumor cells in general appear to be strongly tumor supportive. While approaches are being investigated therapeutically to target these interactions, we have yet to see if these will prove effective in improving patient outcome in PDAC.
VI. CONCLUSIONS
Based on current and past clinical trials and studies, future therapeutic regimens for pancreatic cancer should consider agents targeting critical pathways in the development, progression, and perpetuation of the tumor stroma. Difficulties we have encountered in drug development thus far have been truly instructive. Preclinical work with hyaluronidase suggests that stromal targeting may, in principle, be an effective treatment strategy. Hedgehog signaling inhibition had offered some hope that stromal inhibition can mediate a more effective treatment regimen, yet in a small, randomized phase II trial of one hedgehog-signaling inhibitor, it ultimately proved ineffective at prolonging patient survival.
Moving forward, we must seek a better understanding of the tumor stroma in the specific context of PDAC if we are to develop more effective regimens. The unfortunate failure of bevacizumab in PDAC also highlights this point. Tumor–stromal interactions are many. With a better understanding of the signaling mechanisms most vital in the development of PDAC stroma, we should be better able to design drug development programs more rationally that include stromal-targeting components that will achieve better outcomes for our patients.
Acknowledgments
Research in the authors’ laboratories is supported by grants from the NIH/NCI (CA109552, CA140924, and CA095031), Stand Up to Cancer (SU2C), and by contributions from the Katz Family Foundation, the National Foundation for Cancer Research (NFCR), the Seena Magowitz Foundation, and Jai Pausch.
ABBREVIATIONS
- CAF
cancer-associated fibroblast
- CSF-1
colony stimulating factor 1
- CTLA4
cytotoxic T-lymphocyte antigen 4
- DC
dendritic cell
- DT
diphtheria toxin
- ECM
extracellular matrix
- EF-2
elongation factor 2
- EGF
epidermal growth factor
- EMT
epithelial-to-mesenchymal transition
- FAK
focal adhesion kinase
- HA
hyaluronic acid
- HNSCC
head and neck squamous cell carcinoma
- IGF
insulin-like growth factor
- MMP
matrix metalloproteinase
- NAD
nictotinamide adenine dinucleotide
- NSCLC
non-squamous non-small cell lung cancer
- PDAC
pancreatic ductal adenocarcinoma
- RHAMM
hyaluronan-mediated motility receptor
- ROS
reactive oxygen species
- sCLU
secreted clusterin
- SDF-1
stromal-derived factor 1
- SPARC
secreted protein acidic and rich in cysteine
- TAM
tumor-associated macrophage
- TGFβ
transforming growth factor β
- TP
thymidine phosphorylase
- Treg
regulatory T cell
- TSP-1
thrombospondin-1
- uPA
urokinase plasminogen activator
- VEGF
vascular endothelial growth factor
References
- 1.Paget S. The distribution of secondary growths in cancer of the breast. The Lancet. 1889;133:571–73. [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1,186–97. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 4.Yen TW, Aardal NP, Bronner MP, Thorning DR, Savard CE, Lee SP, Bell RH., Jr Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery. 2002;131:129–34. doi: 10.1067/msy.2002.119192. [DOI] [PubMed] [Google Scholar]
- 5.Magzoub M, Jin S, Verkman AS. Enhanced macromolecule diffusion deep in tumors after enzymatic digestion of extracellular matrix collagen and its associated proteoglycan decorin. FASEB J. 2008;22:276–84. doi: 10.1096/fj.07-9150com. [DOI] [PubMed] [Google Scholar]
- 6.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2,497–2,503. [PubMed] [Google Scholar]
- 7.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–92. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 8.Komar G, Kauhanen S, Liukko K, Seppanen M, Kajander S, Ovaska J, Nuutila P, Minn H. Decreased blood flow with increased metabolic activity: a novel sign of pancreatic tumor aggressiveness. Clin Cancer Res. 2009;15:5,511–17. doi: 10.1158/1078-0432.CCR-09-0414. [DOI] [PubMed] [Google Scholar]
- 9.Reuter SR, Redman HC, Bookstein JJ. Differential problems in the angiographic diagnosis of carcinoma of the pancreas. Radiology. 1970;96:93–99. doi: 10.1148/96.1.93. [DOI] [PubMed] [Google Scholar]
- 10.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–26. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 11.Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226:380–93. doi: 10.1002/path.3000. [DOI] [PubMed] [Google Scholar]
- 12.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1,028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 13.Linder S, Castanos-Velez E, von Rosen A, Biberfeld P. Immunohistochemical expression of extracellular matrix proteins and adhesion molecules in pancreatic carcinoma. Hepatogastroenterology. 2001;48:1,321–27. [PubMed] [Google Scholar]
- 14.Grzesiak JJ, Ho JC, Moossa AR, Bouvet M. The integrin-extracellular matrix axis in pancreatic cancer. Pancreas. 2007;35:293–301. doi: 10.1097/mpa.0b013e31811f4526. [DOI] [PubMed] [Google Scholar]
- 15.Gerson KD, Shearstone JR, Maddula VS, Seligmann BE, Mercurio AM. Integrin beta4 regulates SPARC protein to promote invasion. J Biol Chem. 2012;287:9,835–44. doi: 10.1074/jbc.M111.317727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, Yeo C, Iacobuzio-Donahue C, Goggins M. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–25. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 17.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, Zhang H, Soon-Shiong P, Shi T, Rajeshkumar NV, Maitra A, Hidalgo M. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4,548–54. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matter ML, Ruoslahti E. A signaling pathway from the alpha5beta1 and alpha(v)beta3 integrins that elevates bcl-2 transcription. J Biol Chem. 2001;276:27,757–63. doi: 10.1074/jbc.M102014200. [DOI] [PubMed] [Google Scholar]
- 19.Uhm JH, Dooley NP, Kyritsis AP, Rao JS, Gladson CL. Vitronectin, a glioma-derived extracellular matrix protein, protects tumor cells from apoptotic death. Clin Cancer Res. 1999;5:1,587–94. [PubMed] [Google Scholar]
- 20.Scatena M, Almeida M, Chaisson ML, Fausto N, Nicosia RF, Giachelli CM. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1,083–93. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4,995–5,004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 22.Courter DL, Lomas L, Scatena M, Giachelli CM. Src kinase activity is required for integrin alphaVbeta3-mediated activation of nuclear factor-kappaB. J Biol Chem. 2005;280:12,145–51. doi: 10.1074/jbc.M412555200. [DOI] [PubMed] [Google Scholar]
- 23.Bao W, Stromblad S. Integrin alphav-mediated inactivation of p53 controls a MEK1-dependent melanoma cell survival pathway in three-dimensional collagen. J Cell Biol. 2004;167:745–56. doi: 10.1083/jcb.200404018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci U S A. 1995;92:6,161–65. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, Muthuswamy SK, Brugge JS. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–40. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 26.Hazlehurst LA, Argilagos RF, Dalton WS. Beta1 integrin mediated adhesion increases Bim protein degradation and contributes to drug resistance in leukaemia cells. Br J Haematol. 2007;136:269–75. doi: 10.1111/j.1365-2141.2006.06435.x. [DOI] [PubMed] [Google Scholar]
- 27.Whatcott CJ, Han H, Posner RG, Hostetter G, Von Hoff DD. Targeting the tumor microenvironment in cancer: why hyaluronidase deserves a second look. Cancer Discov. 2011;1:291–96. doi: 10.1158/2159-8290.CD-11-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra S, Heldin P, Hascall VC, Karamanos NK, Skandalis SS, Markwald RR, Ghatak S. Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J. 2011;278:1,429–43. doi: 10.1111/j.1742-4658.2011.08071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung T, Gross W, Zoller M. CD44v6 coordinates tumor matrix-triggered motility and apoptosis resistance. J Biol Chem. 2011;286:15,862–74. doi: 10.1074/jbc.M110.208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meran S, Luo DD, Simpson R, Martin J, Wells A, Steadman R, Phillips AO. Hyaluronan facilitates transforming growth factor-beta1-dependent proliferation via CD44 and epidermal growth factor receptor interaction. J Biol Chem. 2011;286:17,618–30. doi: 10.1074/jbc.M111.226563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, Frost GI, Shepard HM, Skepper JN, Tuveson DA. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2012 doi: 10.1136/gutjnl-2012-302529. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenny PA, Bissell MJ. Tumor reversion: correction of malignant behavior by microenvironmental cues. Int J Cancer. 2003;107:688–95. doi: 10.1002/ijc.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534–41. doi: 10.1136/gut.44.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faouzi S, Le Bail B, Neaud V, Boussarie L, Saric J, Bioulac-Sage P, Balabaud C, Rosenbaum J. Myofibroblasts are responsible for collagen synthesis in the stroma of human hepatocellular carcinoma: an in vivo and in vitro study. J Hepatol. 1999;30:275–84. doi: 10.1016/s0168-8278(99)80074-9. [DOI] [PubMed] [Google Scholar]
- 37.Cullen KJ, Smith HS, Hill S, Rosen N, Lippman ME. Growth factor messenger RNA expression by human breast fibroblasts from benign and malignant lesions. Cancer Res. 1991;51:4,978–85. [PubMed] [Google Scholar]
- 38.Naber HP, ten Dijke P, Pardali E. Role of TGF-beta in the tumor stroma. Curr Cancer Drug Targets. 2008;8:466–72. doi: 10.2174/156800908785699342. [DOI] [PubMed] [Google Scholar]
- 39.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–20. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 40.Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 41.Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–3. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 42.Blackford A, Serrano OK, Wolfgang CL, Parmigiani G, Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Eshleman JR, Goggins M, Jaffee EM, Iacobuzio-Donahue CA, Maitra A, Cameron JL, Olino K, Schulick R, Winter J, Herman JM, Laheru D, Klein AP, Vogelstein B, Kinzler KW, Velculescu VE, Hruban RH. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res. 2009;15:4,674–9. doi: 10.1158/1078-0432.CCR-09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, Vilardell F, Wang Z, Keller JW, Banerjee P, Herman JM, Cameron JL, Yeo CJ, Halushka MK, Eshleman JR, Raben M, Klein AP, Hruban RH, Hidalgo M, Laheru D. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1,806–13. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yi JY, Shin I, Arteaga CL. Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J Biol Chem. 2005;280:10,870–6. doi: 10.1074/jbc.M413223200. [DOI] [PubMed] [Google Scholar]
- 45.Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2,008–11. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 47.Mulder KM, Morris SL. Activation of p21ras by transforming growth factor beta in epithelial cells. J Biol Chem. 1992;267:5,029–31. [PubMed] [Google Scholar]
- 48.Muraoka-Cook RS, Kurokawa H, Koh Y, Forbes JT, Roebuck LR, Barcellos-Hoff MH, Moody SE, Chodosh LA, Arteaga CL. Conditional overexpression of active transforming growth factor beta1 in vivo accelerates metastases of transgenic mammary tumors. Cancer Res. 2004;64:9,002–11. doi: 10.1158/0008-5472.CAN-04-2111. [DOI] [PubMed] [Google Scholar]
- 49.Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J, Easterly E, Roebuck LR, Ryan S, Gotwals PJ, Koteliansky V, Arteaga CL. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1,551–9. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang YA, Dukhanina O, Tang B, Mamura M, Letterio JJ, MacGregor J, Patel SC, Khozin S, Liu ZY, Green J, Anver MR, Merlino G, Wakefield LM. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002;109:1,607–15. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zion O, Genin O, Kawada N, Yoshizato K, Roffe S, Nagler A, Iovanna JL, Halevy O, Pines M. Inhibition of transforming growth factor beta signaling by halofuginone as a modality for pancreas fibrosis prevention. Pancreas. 2009;38:427–35. doi: 10.1097/MPA.0b013e3181967670. [DOI] [PubMed] [Google Scholar]
- 52.Adrian K, Strouch MJ, Zeng Q, Barron MR, Cheon EC, Honasoge A, Xu Y, Phukan S, Sadim M, Bentrem DJ, Pasche B, Grippo PJ. Tgfbr1 haploinsufficiency inhibits the development of murine mutant Kras-induced pancreatic precancer. Cancer Res. 2009;69:9,169–74. doi: 10.1158/0008-5472.CAN-09-1705. [DOI] [PubMed] [Google Scholar]
- 53.Nagashio Y, Ueno H, Imamura M, Asaumi H, Watanabe S, Yamaguchi T, Taguchi M, Tashiro M, Otsuki M. Inhibition of transforming growth factor beta decreases pancreatic fibrosis and protects the pancreas against chronic injury in mice. Lab Invest. 2004;84:1,610–8. doi: 10.1038/labinvest.3700191. [DOI] [PubMed] [Google Scholar]
- 54.Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004;28:38–44. doi: 10.1097/00006676-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis. 2008;25:593–600. doi: 10.1007/s10585-008-9143-9. [DOI] [PubMed] [Google Scholar]
- 56.Postigo AA. Opposing functions of ZEB proteins in the regulation of the TGFbeta/BMP signaling pathway. EMBO J. 2003;22:2,443–52. doi: 10.1093/emboj/cdg225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haddad Y, Choi W, McConkey DJ. Delta-crystallin enhancer binding factor 1 controls the epithelial to mesenchymal transition phenotype and resistance to the epidermal growth factor receptor inhibitor erlotinib in human head and neck squamous cell carcinoma lines. Clin Cancer Res. 2009;15:532–42. doi: 10.1158/1078-0432.CCR-08-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gold DV, Karanjawala Z, Modrak DE, Goldenberg DM, Hruban RH. PAM4-reactive MUC1 is a biomarker for early pancreatic adenocarcinoma. Clin Cancer Res. 2007;13:7,380–7. doi: 10.1158/1078-0432.CCR-07-1488. [DOI] [PubMed] [Google Scholar]
- 59.Gold DV, Modrak DE, Ying Z, Cardillo TM, Sharkey RM, Goldenberg DM. New MUC1 serum immunoassay differentiates pancreatic cancer from pancreatitis. J Clin Oncol. 2006;24:252–8. doi: 10.1200/JCO.2005.02.8282. [DOI] [PubMed] [Google Scholar]
- 60.Ocean AJ, Pennington KL, Guarino MJ, Sheikh A, Bekaii-Saab T, Serafini AN, Lee D, Sung MW, Gulec SA, Goldsmith SJ, Manzone T, Holt M, O’Neil BH, Hall N, Montero AJ, Kauh J, Gold DV, Horne H, Wegener WA, Goldenberg DM. Fractionated radioimmunotherapy with (90) Y-clivatuzumab tetraxetan and low-dose gemcitabine is active in advanced pancreatic cancer: A phase 1 trial. Cancer. 2012 doi: 10.1002/cncr.27592. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tremblay GB, Viau E, Filion M. The EMT inhibitor AB-16B5 interacts with specific isoforms of secreted clusterin. Cancer Res. 2012;72(8 Suppl):Abstract nr LB-297. [Google Scholar]
- 62.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 63.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 64.Jirillo A, Vascon F, Giacobbo M. Bevacizumab in advanced cancer, too much or too little? Ann Oncol. 2008;19:1,817–8. doi: 10.1093/annonc/mdn564. [DOI] [PubMed] [Google Scholar]
- 65.Gemcitabine plus bevacizumab no better than gemcitabine alone in clinical study of 600 patients with pancreatic cancer. Cancer Biol Ther. 2007;6:132–140. [PubMed] [Google Scholar]
- 66.Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, Moore MJ. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2,231–7. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 67.Whipple C, Korc M. Targeting angiogenesis in pancreatic cancer: rationale and pitfalls. Langenbecks Arch Surg. 2008;393:901–10. doi: 10.1007/s00423-008-0280-z. [DOI] [PubMed] [Google Scholar]
- 68.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1,457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–11. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 70.Kusano KF, Allendoerfer KL, Munger W, Pola R, Bosch-Marce M, Kirchmair R, Yoon YS, Curry C, Silver M, Kearney M, Asahara T, Losordo DW. Sonic hedgehog induces arteriogenesis in diabetic vasa nervorum and restores function in diabetic neuropathy. Arterioscler Thromb Vasc Biol. 2004;24:2,102–7. doi: 10.1161/01.ATV.0000144813.44650.75. [DOI] [PubMed] [Google Scholar]
- 71.Perez-Mancera PA, Guerra C, Barbacid M, Tuveson DA. What we have learned about pancreatic cancer from mouse models. Gastroenterology. 2012;142:1,079–92. doi: 10.1053/j.gastro.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 72.Madden JI. Infinity Reports Update from Phase 2 Study of Saridegib Plus Gemcitabine in Patients with Metastatic Pancreatic Cancer. 2012 Available from: http://phx.corporate-ir.net/phoenix.zhtml?c=121941&p=irol-newsArticle&ID=1653550&highlight=
- 73.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 74.Badawi AF, Mostafa MH, Probert A, O’Connor PJ. Role of schistosomiasis in human bladder cancer: evidence of association, aetiological factors, and basic mechanisms of carcinogenesis. Eur J Cancer Prev. 1995;4:45–59. doi: 10.1097/00008469-199502000-00004. [DOI] [PubMed] [Google Scholar]
- 75.Williams MP, Pounder RE. Helicobacter pylori: from the benign to the malignant. Am J Gastroenterol. 1999;94:S11–6. doi: 10.1016/s0002-9270(99)00657-7. [DOI] [PubMed] [Google Scholar]
- 76.McCance DJ. Human papillomaviruses and cancer. Biochim Biophys Acta. 1986;823:195–205. doi: 10.1016/0304-419x(86)90002-8. [DOI] [PubMed] [Google Scholar]
- 77.Lowenfels AB, Maisonneuve P, DiMagno EP, Elitsur Y, Gates LK, Jr, Perrault J, Whitcomb DC. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442–6. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 78.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 79.Knaapen AM, Gungor N, Schins RP, Borm PJ, Van Schooten FJ. Neutrophils and respiratory tract DNA damage and mutagenesis: a review. Mutagenesis. 2006;21:225–36. doi: 10.1093/mutage/gel032. [DOI] [PubMed] [Google Scholar]
- 80.Tuo J, Wolff SP, Loft S, Poulsen HE. Formation of nitrated and hydroxylated aromatic compounds from benzene and peroxynitrite, a possible mechanism of benzene genotoxicity. Free Radic Res. 1998;28:369–75. doi: 10.3109/10715769809070805. [DOI] [PubMed] [Google Scholar]
- 81.Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, Cañamero M, Rodriguez-Justo M, Serrano M, Barbacid M. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–39. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Negus RP, Stamp GW, Hadley J, Balkwill FR. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol. 1997;150:1,723–34. [PMC free article] [PubMed] [Google Scholar]
- 83.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9,518–27. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 84.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2,224–34. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 85.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta. 2009;1796:11–18. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 86.Martin-Manso G, Galli S, Ridnour LA, Tsokos M, Wink DA, Roberts DD. Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells. Cancer Res. 2008;68:7,090–9. doi: 10.1158/0008-5472.CAN-08-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–8. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 88.Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS, Bicknell R, Taylor M, Gatter KC, Harris AL. Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in Human breast cancer. Cancer Res. 2002;62:1,326–9. [PubMed] [Google Scholar]
- 89.Hildenbrand R, Jansen C, Wolf G, Bohme B, Berger S, von Minckwitz G, Hörlin A, Kaufmann M, Stutte HJ. Transforming growth factor-beta stimulates urokinase expression in tumor-associated macrophages of the breast. Lab Invest. 1998;78:59–71. [PubMed] [Google Scholar]
- 90.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. 1998;28:359–69. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 91.Schmid-Kotsas A, Gross HJ, Menke A, Weidenbach H, Adler G, Siech M, Beger H, Grünert A, Bachem MG. Lipopolysaccharide-activated macrophages stimulate the synthesis of collagen type I and C-fibronectin in cultured pancreatic stellate cells. Am J Pathol. 1999;155:1,749–58. doi: 10.1016/S0002-9440(10)65490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12,493–8. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, Sendo F, Kobayashi M, Hosokawa M. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol. 2003;163:2,221–32. doi: 10.1016/S0002-9440(10)63580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2,756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 95.Liyanage UK, Goedegebuure PS, Moore TT, Viehl CT, Moo-Young TA, Larson JW, Frey DM, Ehlers JP, Eberlein TJ, Linehan DC. Increased prevalence of regulatory T cells (Treg) is induced by pancreas adenocarcinoma. J Immunother. 2006;29:416–24. doi: 10.1097/01.cji.0000205644.43735.4e. [DOI] [PubMed] [Google Scholar]
- 96.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1,057–61. [PubMed] [Google Scholar]
- 97.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–71. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 98.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 99.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 100.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–16. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 101.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–33. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foss FM. DAB(389)IL-2 (ONTAK): a novel fusion toxin therapy for lymphoma. Clin Lymphoma. 2000;1:110–6. doi: 10.3816/clm.2000.n.009. discussion 7. [DOI] [PubMed] [Google Scholar]
- 103.Jones E, Dahm-Vicker M, Simon AK, Green A, Powrie F, Cerundolo V, Gallimore A. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2002;2:1–12. [PubMed] [Google Scholar]
- 104.Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32:3,267–75. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 105.Talpur R, Apisarnthanarax N, Ward S, Duvic M. Treatment of refractory peripheral T-cell lymphoma with denileukin diftitox (ONTAK) Leuk Lymphoma. 2002;43:121–6. doi: 10.1080/10428190210183. [DOI] [PubMed] [Google Scholar]
- 106.McGinnis KS, Shapiro M, Junkins-Hopkins JM, Smith M, Lessin SR, Vittorio CC, Rook AH. Denileukin diftitox for the treatment of panniculitic lymphoma. Arch Dermatol. 2002;138:740–2. doi: 10.1001/archderm.138.6.740. [DOI] [PubMed] [Google Scholar]
- 107.Kreitman RJ. Recombinant immunotoxins containing truncated bacterial toxins for the treatment of hematologic malignancies. BioDrugs. 2009;23:1–13. doi: 10.2165/00063030-200923010-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880–7. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 109.Filmus J. Glypicans in growth control and cancer. Glycobiology. 2001;11:19R–23R. doi: 10.1093/glycob/11.3.19r. [DOI] [PubMed] [Google Scholar]
- 110.Ishiwata T, Cho K, Kawahara K, Yamamoto T, Fujiwara Y, Uchida E, Tajiri T, Naito Z. Role of lumican in cancer cells and adjacent stromal tissues in human pancreatic cancer. Oncol Rep. 2007;18:537–43. [PubMed] [Google Scholar]
- 111.Stern R. Association between cancer and “acid mucopolysaccharides”: an old concept comes of age, finally. Semin Cancer Biol. 2008;18:238–43. doi: 10.1016/j.semcancer.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 112.Marastoni S, Ligresti G, Lorenzon E, Colombatti A, Mongiat M. Extracellular matrix: a matter of life and death. Connect Tissue Res. 2008;49:203–6. doi: 10.1080/03008200802143190. [DOI] [PubMed] [Google Scholar]
- 113.Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC, Strieter R, Haslett C. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5:662–8. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 114.Ricciardelli C, Sakko AJ, Ween MP, Russell DL, Horsfall DJ. The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev. 2009;28:233–45. doi: 10.1007/s10555-009-9182-y. [DOI] [PubMed] [Google Scholar]
- 115.Shimizu K. Mechanisms of pancreatic fibrosis and applications to the treatment of chronic pancreatitis. J Gastroenterol. 2008;43:823–32. doi: 10.1007/s00535-008-2249-7. [DOI] [PubMed] [Google Scholar]
- 116.Kobayashi H, Boelte KC, Lin PC. Endothelial cell adhesion molecules and cancer progression. Curr Med Chem. 2007;14:377–86. doi: 10.2174/092986707779941032. [DOI] [PubMed] [Google Scholar]