Abstract
Background: This work aimed to show and compare the degradation time of some of cartilage extracellular matrix components using an in vitro model for cartilage degradation induced by interleukin-1α. It is known that elucidation of molecular events under Interleukin-1α induction of bovine nasal cartilage could obtain useful data to understand more about involving mechanisms for tissue breakdown in joint disease. Methods: The cartilage was taken from an adult bovine in a local slaughterhouse. After removing the whole perichondrium, the equal 2 mm diameter pieces of bovine nasal cartilage were punched out and cultured in Dulbecco's modified Eagle's medium DMEM with or without 10 ng/ml Interleukin-1α for 24 days. Each 3 days, the media were removed and exchanged by fresh media, and the removed media were stored in -20C. Sodium dodecyl sulphate/polyacrylamid-gel electrophoresis SDS-PAGE and Western-blot methods were used for analyzing the samples. Results: The first fragment of fibromodulin (FM) was seen at day 6 and further fragments were appeared at day 18. Cartilage oligomeric matrix protein (COMP) releasing was as a successive pattern during culture period and the first fragment was found at day 6. Collagen IX fragments were seen at day 9 and in a progressive pattern until the end of the study. Conclusion: This study shows that FM and COMP could be considered as the suitable candidates for studying the mechanisms that participate in the cartilage degradations.
Key Words: Interleukin-1α, Bovine cartilage, Cartilage oligomeric matrix protein (COMP), Collagen IX, Fibromodulin (FM)
INTRODUCTION
Cartilage, a special connective tissue, contains one cell type, the chondrocyte, producing an abundant extracellular matrix (ECM). ECM consists of a large number of molecules such as collagens, aggrecan and non-collagenous proteins that interact to form complex structures needed for tissue properties [1]. Chondrocytes have a key role in matrix turnover and maintaining the structural integrity in response to the load changes or material fatigue which result due to repeated mechanical stress [2].
Rheumatoid arthritis (RA) as a joint disease affects about 1% of population [3, 4]. Destruction of articular cartilage and underlying bone is a major indication in this disease [5].
Normally, there is a balance between synthesis and degradation of ECM macromolecules while in RA this balance is changed and shifted towards degradation. This process finally leads to disintegration of the cartilage tissue and eventually the involved joint. The integrity of the ECM plays a key role in the normal function of articular cartilage which primarily involves taking up and distributing of load [5, 6]. Processes leading ECM disintegrity result in an increase in macromolecules and fragmentations which could be released in synovial fluid or even blood and urine. By measuring these macromolecules or fragments (biomarkers) in patient's body fluid such as serum, it will be possible to identify the activity of the process and consequently to manage an effective therapy to prevent further destruction [5, 7-9].
Fibromodulin (FM) and cartilage oligomeric matrix protein (COMP) are two members of non-collagenous protein components of cartilage. Both proteins could be released into synovial fluid when destruction of articular cartilage is occured. FM is a member of leucine rich-repeat protein family which its functions are gradually being unraveled [10]. The core protein of FM is 59 kDa. This molecule contains four asparagines residues in the central domain where either N-linked oligosaccharide or N-linked keratan sulfate chain substitution occurs [11]. FM binds to collagen I and II and has a role in modulating fiber formation [12].
COMP is a member of thrombospondin (TSP) family which is also called TSP-5. This pentameric protein is composed of five identical subunits with MW of 86.5 ± 16.3 kDa [13]. The subunits near the N-terminus are joined by a coiled-coil domain while there is a globular domain in the C-terminus and involves in interactions with other matrix components of cartilage [14,15]. By interacting with collagen I and thin fibrils, COMP increases the rate and efficiency of collagen II fibril formation [15].
Collagen type IX is belonging to fibril associated collagens with interrupted triple helices family (FACIT) [16]. This collagen has three typical COL domains (COL1-3) which are surrounded by four non-collagen (NC) domains (NC 1-4). The molecule is a heterotrimer formed by three different α chain (α1-α2 and α3) [17]. This type of collagen by decoration of collagen II surface probably has an important role in interconnecting of cartilage tissue [16].
Since during destruction process these macromolecules come out from cartilage, identifying them or other macromolecules could be useful to understand the underlying process. There is little data about which proteins of the structural elements are first degraded and what degradation events follows. The aim of this study was to assess the sequence of degradation events in selected ECM macromolecules to elucidate the mechanisms of tissue breakdown in joint disease. The beginning of disease process and its proper development are still unknown. We, therefore, assessed the fragmentation of FM, COMP, and collagen type IX by using a well-defined in vitro model which has been already applied [9, 17-19].
MATERIALS AND METHODS
Tissue culture. Bovine nasal septum was obtained from an adult animal from a local slaughterhouse shortly after sacrifice. For tissue culture studies, the cartilage was separated from the whole septum and the perichondrium was removed carefully by a scalpel knife as much as possible [20]. The cartilage was thoroughly washed with sterile PBS and then with washing solution containing Dulbecco's modified Eagle's medium DMEM with ×10 PEST (1000 U/ml Penicillin, 1000 µg/ml streptomycin sulphate) and finally followed by washing with DMEM containing ×1 PEST for 5 times. Pieces of cartilage (2 mm diameter) were punched aseptically by full-depth core and equally divided and placed approximately 90 mg of tissue in each well (sterile plate Costar 24-well). The pieces were pre-cultured for one day in DMEM containing 100µg/ml tissue culture grade BSA) (Sigma, Sweden) and 50µg/ml ascorbic acid 2-phosphate (Vit. C) (Sigma, Sweden) [17]. The next day, as the day 0, the pre-culture medium was substituted by fresh DMEM containing 100 µg/ml tissue culture grade BSA and 25 µg/ml Vit.C in the presence of 10 ng/ml IL-1α as test group and in the absence of IL-1α as control group. The removed medium was collected and stored at -20°C. This procedure was repeated every 3 days until day 24. The explants in the test group were gradually started to change to loose and translucent pieces from day 18 and at day 24, they were completely loosed and their handling was difficult. At day 24, culture was ended and the explants were harvested totally.
Digestion of medium. In order to eliminate interference of keratan sulphate and N-linked oligosaccharides chains and visualisation of core protein of FM, the media were treated with a final concentration of 10 mU/ml N-Glycosidase F (Boehringer-Mannheim). Two µl of the enzyme by concentration of 100 mU/ml was added to 20 µl of samples and incubated at 37°C overnight [20, 21]. Then, the samples were prepared for electrophoresis.
SDS-PAGE. The media samples from both control and IL-1α-treated groups were thawed in room temperature. Then, 20 µl of samples were dissolved in the sample buffer (modified Laemmli) containing 0.125 M Tris HCl, 5% SDS, 20% glycerol and bromophenol blue with or without 2 mM β-mercaptoethanol (for reducing some samples) [22] and mixed shortly. The samples were heated in a boiling water bath for 2 minutes. Then a short spin down was applied and the samples were loaded in a 10% linear tricine gel with a 4% stacking gel and the buffer system [23]. The gels were run with 60V for 4-4.5 hours.
Western-blotting. After electrophoresis on 10% linear tricine gels, samples were transferred to a polyvinylidene fluoride membrane as reported by Towbin [24]. Then, the membranes were incubated in Tris buffered saline (pH 7.4) containing 0.05 v/v Tween 20 (Sigma/Sweden) TBST at 4°C overnight. For detection of different proteins, a panel of various antibodies was applied. A polyclonal rabbit anti-bovine FM [20], a polyclonal rabbit full length protein anti-bovine COMP (α-92) [14] and an anti-peptide QCG-16 for the NC-4 domain of collagen IX were applied [17]. The antibodies were diluted in 3% BSA/TBST (1:1000) and the membranes were incubated by continuous agitation for 1 hour. The secondary antibody, horseradish phosphate-conjugated rabbit anti-swine antibody (DAKO, P0217, Denmark) diluted 1:5000 in 3% BSA/TBST, was used and the membrane was incubated for 1 h. The membranes were then incubated with the home-made ECL solution for 1 minute and were exposed to Agfa CRONEX® medical x-ray film (Sterling Diagnostic Imaging, USA) at a dark room for various durations.
RESULTS
In this study, we employed a bovine cartilage culture model to find out and compare the degradation of collagen IX, FM and COMP to each other.
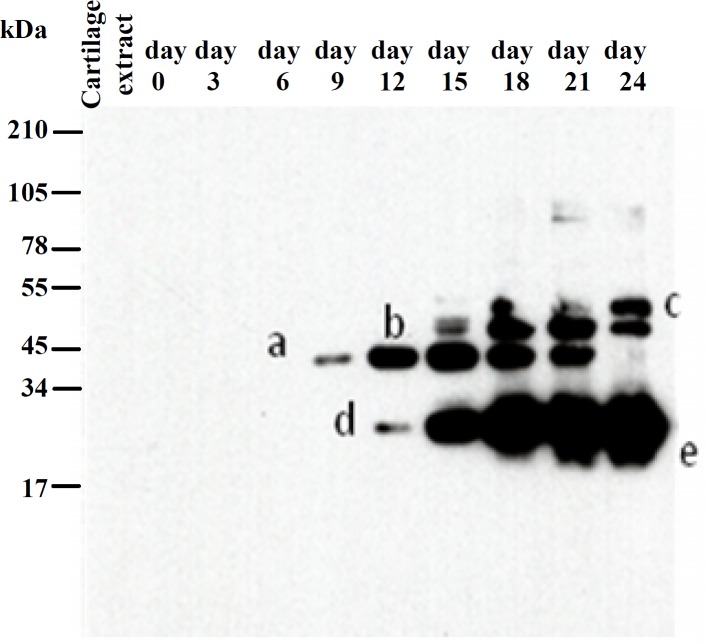
Collagen IX detection. Day 9 was the earliest time to detect collagen IX in test group. In this time point of culture a fragment at the position of 34-45 kDa (band a) was delivered into the medium with a progressive pattern until day 21 of culture and a deletion subsequently. Another fragment with 55 kDa (band b) was appeared at the day 15 and remained constantly until 24th day. A fragment (band c) bigger than 55 kDa was detectable during days 18-24. There was another band at the position between 17-34 kDa and the shape of this band showed at least two different size fragments, both with a successive releasing pattern. The bigger one (band d) was released from day 12 and the smaller one (band e) from day 18 of culture to the end of the experiment (Fig. 1). The figure of separated proteins by SDS-PAGE in the control group showed collagen IX remains stable in the absence of IL-1α and neither whole molecule nor any fragments were appeared as a consequence of induction by IL-1α (figure was blank and data not shown).
Fig. 1.
Western-blot with an anti-peptide QCG-16 for collagen IX in IL-1α treated cartilage explants. Media from days 0, 3, 6, 9, 12, 15, 18, 21 and 24 of IL-1α treated cartilage were separated by SDS-PAGE on 10% linear tricine gel and transferred to a polyvinylidene fluoride membrane. Position of collagen IX and its fragments was determined by using the NC4 domain antiserum. Produced fragments are shown by a, b, c, d and e related to their specific time releasing. The control gel was not shown here since no band was detected on this group gel.
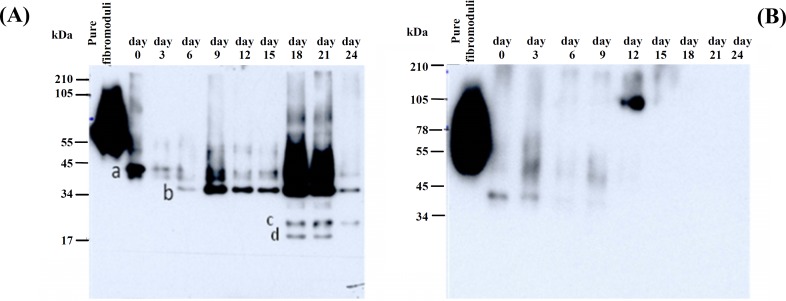
Fibromodulin detection. In the presence of IL1-α, intact FM with a 59 kDa core protein and its fragments were released into the medium. As shown in Figure 2, deglycosylated intact FM (band a) migrated close to 50 kDa as a weak band and remained permanently weak until the end of the experiment while another fragment (band b) was appeared at position 34 kDa on day 6 and remained more prominent by the time especially at days 18 and 21 and became weaker in the end of trial. Furthermore, two more fragments (bands c and d) smaller than the previous one between 22-34 kDa were detectable only at days 18 and 21 by this difference that the smaller one (band d) is disappeared at day 24. Unlike test group, in the control group, just intact FM was released on the days 0 and 3 and after that time point no fragment neither intact protein came out into the medium (Fig. 2).
Fig. 2.
Western-blot with a polyclonal rabbit anti-bovine fibromodulin antibody in IL-1α treated (A) and control (B) cartilage explants. Media from days 0, 3, 6, 9, 12,15,18, 21 and 24 of both IL-1α treated and control cartilage were separated by SDS-PAGE on 10% linear tricine gel and transferred to a polyvinylidene fluoride membrane. Position of fibromodulin fragments was determined by using a polyclonal rabbit anti-bovine fibromodulin antibody specific for its core protein. The letter a, b, c and d indicate the position of produced fragments related to their specific time releasing.
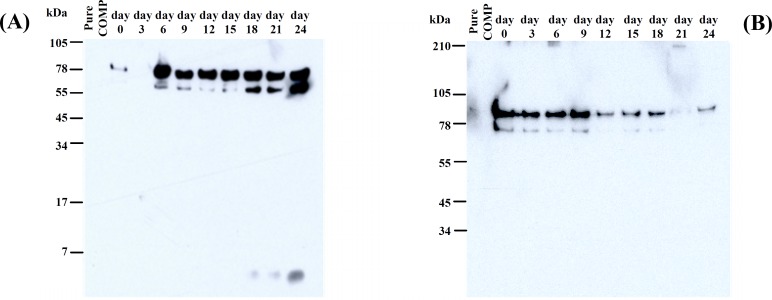
COMP detection. Results obtained with western-blot in both reduced and non-reduced gels (data not shown) revealed IL-1α, by affecting on explants, produced the fragments which never seen in control medium. Compared COMP degradation pattern in reduced IL-1α treated and control group medium, in the IL-1α treated culture two fragments at position 55-78 and 78-105 kDa were gradually increased by a time point pattern, whereas in the control sample, 78-105 kDa band significantly diminished and the smaller band was detected from the first day until
the day 9 and after that it was completely disappeared. In addition, a fragment smaller than 7 kDa was seen on the day 18 in the IL-1α treated group by a noticeable feature on the day 24 (Fig. 3).
Fig. 3.
Western-blot with a polyclonal rabbit full length protein anti-bovine COMP (α-92) in IL-1α treated (A) and control (B) cartilage explants. Media from days 0, 3, 6, 9, 12, 15, 18, 21 and 24 of IL-1α treated cartilage were separated by SDS-PAGE on 10% linear tricine gel and transferred to a polyvinylidene fluoride membrane. Location of COMP monomers and its fragments were determined by using a polyclonal rabbit full length protein anti-bovine COMP (α-92).
DISCUSSION
In the current work, for studying cartilage macromolecule degradation which is induced by RA disease, we used an in vitro RA model by treatment cartilage explants with IL-1α as were previously used by many investigators [17, 19, 20]. Results in this study shows the FM is degraded due to IL-1α and some fragments by different molecular weight released from cartilage into the medium. Keratan sulphate and N-linked oligosaccharides along with FM make a dispersive pattern during gel electrophoresis. Therefore, to obtain a relatively sharp band of FM core protein, we used N- glycosidase digestion followed by SDS-PAGE as a useful approach. When deglycosylation is performed, intact FM runs close to 50 kDa and one of the fragments migrated around 34 kDa, this fragment is reported by the another group as well [20]. The 34 kDa fragment is released into the medium on the day 6 of the experiment, the same time as COMP released. Time releasing data is similar to data exclaimed COMP and FM are released at the same time [20]. In this experience two other fragments are detected in the IL-1α treated medium, both of them are smaller than 34 kDa and are appeared in late period of culture, one of them is seen at day 18-24, while the another one is found
only from day 18 to 21. These latter fragments indicate progressive degradation induced by IL-1α over the time. Existence of intact FM in control group at early time probably shows a primary degradation under the culture condition which stops completely after that short period releasing.
The bands belong to collagen IX at position 34-45 and 17-34 kDa are the same as bands detected by Danfelter et al. [17], while both the bands around 55 kDa and one smaller than 28 kDa were detected on the day 18 and remained the same until the end of the culture. These fragments showed continuous degradation of collagen IX in the time-dependent manner and may help to elucidate more details about how collagen IX molecule is released into the surrounding space.
A progressive pattern of COMP destruction in the presence of IL-1α was observed, whereas in the control group, COMP releasing is performed in the starting of the culture until the day 9 and then disappeared gradually. This may indicate a precocious trauma culturing under this condition or as Sally et al. [19] suggested that it is because of COMP loosely bounding within the articular cartilage matrix or existence of unidentified components which challenge for COMP binding site in this tissue. The difference between COMP bands on the days 0 and 3 in both groups is probably due to an artifact that may need more assessments. Moreover, releasing of COMP monomers in IL-1α treated group, another COMP fragment smaller than 7 kDa, is detectable on the day 18 with a higher amount at the last day of harvest period. It may lead us to new data related to COMP fragmentation.
All these fragments could provide a partial explanation for destabilizing of connections between ECM macromolecules and loss of tissue integrity due to cytokines such as IL-1α which produces in RA. RA by out breaking joints in early stages may induce a progressive destruction of cartilage structure and disintegrate in ECM and ultimately leads to loss of joint function and disability [25]. By identification and characterization of these molecular fragments and their time of appearance we would gain information related to the molecular events of the process. Elucidation of basic mechanisms in pathogenesis of this disorder, could lead to invent some new approaches to diagnose this disease in early stages before occurring irreversible changes in articular cartilage and joints.
We know the sequence degradation in cartilage treated by IL-1α starts with aggrecan, followed by proteolysis of non-collagenous molecules at intermediate time and then by collagen network [2]. According to our findings in this study, FM and COMP are degraded at the same time, day 6 of culture point, and earlier than collagen IX. This is not the same as previous studies which have acclaimed COMP I s released earlier than FM [17, 20]. Furthermore, COMP releasing in this experience is also earlier than Sally's data which exclaimed COMP is come out from cartilage on the second week of the culture [19].
In line with the role of COMP in binding to collagen type II via collagen IX and also binding of FM to collagen II, degradation of the components under IL-1α condition may involve whole collagen network and lead it toward total tissue degradation as seen obviously in day 21 of the culture. It appears that a time-related degradation of interacting molecules with collagen network in cartilage ECM could be a critical process to clarify basic mechanisms in pathogenesis of RA disease. Finally a concluding remark from this study is that FM and COMP could be considered as the suitable candidates for studying the mechanisms that participate in the cartilage degradations. Thus studying of another non-collagenous ECM molecules and comparing their time-related degradation could be avail in elucidation of participating mechanisms in the joint diseases.
References
- 1.Hardingham T.E. Proteoglycans and Glycol-saminoglycans. In: Seibel M.J., Robins S.P., Brik D.E., editors. Dynamic of Bone and Cartilage Metabolism. USA: Academic Press; 2006. [Google Scholar]
- 2.Heinegard D. Cartilage matrix destruction. In: Bronner F., Carson M.F., editors. Bone and Osteoarthritis. Guildford, UK: Springer; 2007. pp. 79–93. [Google Scholar]
- 3.Englund M., Jud A., Geborek P., Felson D.T., Jacobsson L.T., Petersson I.F. Prevalence and incidence of rheumatoid arthritis in southern Sweden 2008 and their relation to prescribed biologics. Rheumatology J. 2010;49:1563–1569. doi: 10.1093/rheumatology/keq127. [DOI] [PubMed] [Google Scholar]
- 4.Harris E.D. Clinical Features of Rheumatoid Arthritis. In: Harris E.D., Budd R.C., Firestein G.S., Genovese M.C., Serrgent J.S., Ruddy S., Sledge C.B., editors. Kelly's Textbook of Rheumatology. USA: Elsevier Saunders; 2005. pp. 915–950. [Google Scholar]
- 5.Saxne T., Mansson B., Heinegard D. Biomarkers for Cartilage and Bone in Rheumatoid Arthritis. In: Firestein G.S., Panayi G.S., Wollheim F.A., editors. Rheumatoid Arthritis: New Frontiers in Pathogenesis and treatment. (Firestein. Oxford. UK: Oxford University Press; 2006. pp. 301–313. [Google Scholar]
- 6.Mansson B., Carey D., Alini M., Lonescu M., Rosenberg L.C., Poole A.R., Heinegard D., Saxne T. Cartilage and bone metabolism in rheumatoid arthritis: Differences between rapid and slow progression of disease identified by serum markers of cartilage metabolism. J. Clin. Invest. 1995;95:1071–1077. doi: 10.1172/JCI117753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milner J.M., Rowan A.D., Cawston T.E., Young D.A. Metalloproteinase and inhibitor expression profiling of resorbing cartilage reveals pro-collagenase activation as a critical step for collagenolysis. Arthritis Res. Ther. 2006;8:R142. doi: 10.1186/ar2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinegard D., Lorenzo P., Saxne T. Matrix Glycoproteins and Proteoglycans in Cartilage. In: Harris E.D., Budd R.C., Firestein G.S., Genovese M.C., Sergent J.S., Ruddy S., Sledge C.B., editors. Kelly's textbook of rheumatology. Elsevier Saunders; 2005. pp. 48–62. [Google Scholar]
- 9.Dodge G.R., Poole A.R. Immuno-histochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid and osteoarthritic articular cartilage and in explants of Bovine articular cartilage with Interleukin-1. J. Clin. Invest. 1989;83:647–661. doi: 10.1172/JCI113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollander A.P., Heathfield T.F., Webber C., Iwata Y., Bourne R., Rorabeck C., Poole A.R. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J. Clin. Invest. 1994;93:1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danielson K.G., Fazzio A., Cohen I., Cannizzaro L.A., Eichstetter I., Lozzo R.V. The human decorin gene: intron-exon organization, discovery of two alternatively spliced exons in the 5' untranslated region, and mapping of the gene to chromosome 12q23. Genomics. 1993;15:146–160. doi: 10.1006/geno.1993.1022. [DOI] [PubMed] [Google Scholar]
- 12.Hedbom E., Heinegard D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J. Biol. Chem. 1989;264:6898–6905. [PubMed] [Google Scholar]
- 13.Zaia J., Boynton R.E., McIntosh A., Marshak D.R., Olsson H., Heinegard D., Barry F.P. Post-translational modifications in cartilage oligo-meric matrix protein. Characterization of the N-linked oligosaccharides by matrix-assisted laser desorption ionization time-of-flight mass spectro-metry. J. Biol. Chem. 1997;272:14120–14126. doi: 10.1074/jbc.272.22.14120. [DOI] [PubMed] [Google Scholar]
- 14.Morgelin M., Heinegard D., Engel J., Paulsson M. Electron microscopy of native cartilage oligomeric matrix protein purified from the Swarm rat chondrosarcoma reveals a five-armed structure. J. Biol. Chem. 1992;267:6137–6141. [PubMed] [Google Scholar]
- 15.Rosenberg K., Olsson H., Morgelin M., Heinegard D. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J. Biol Chem. 1998;273:20397–20403. doi: 10.1074/jbc.273.32.20397. [DOI] [PubMed] [Google Scholar]
- 16.Shaw L.M., Olsen B.R. FACIT collagens: diverse molecular bridges in extracellular matrices. Trends Biochem. Sci. 1991;16:191–194. doi: 10.1016/0968-0004(91)90074-6. [DOI] [PubMed] [Google Scholar]
- 17.Danfelter M., Onnerfjord P., Heinegard D. Fragmentation of Proteins in Cartilage Treated with Interleukin-1, Specific Cleavage of Type IX Collagen by Matrix Metalloproteinase 13 Releases The NC4 Domain. J. Biol. Chem. 2007;282:36933–36941. doi: 10.1074/jbc.M702491200. [DOI] [PubMed] [Google Scholar]
- 18.Ganu V., Goldberg R., Peppard J., Rediske J., Melton R., Hu S.I., Weigwang W., Duvander C., Heinegard D. Inhibition of interleukin-1α-induced cartilage oligomeric matrix protein degradation in bovine articular cartilage by matrix metalloproteinase inhibitors. Arthritis Rheum. 1998;41:2143–2151. doi: 10.1002/1529-0131(199812)41:12<2143::AID-ART9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Dickinson S.C., Vankemmelbeke M.N., Buttle D.J., Rosenberg K., Heinegard D., Hollander A.P. Cleavage of cartilage oligomeric matrix protein (thrombospondin-5) by matrix metallo-proteinases and a disintegrin and metalloproteinase with thrombospondin motifs. Matrix Biol. 2010;22:267–278. doi: 10.1016/s0945-053x(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 20.Heathfield T.F., Onnerfjord P., Dahlberg L., Heinegard D. Cleavage of fibromodulin in cartilage explants involves removal of the N-terminal tyrosine sulfate-rich region by proteolysis at a site that is sensitive to matrix metalloproteinase-13. J. Biol. Chem. 2004;279:6286–6295. doi: 10.1074/jbc.M307765200. [DOI] [PubMed] [Google Scholar]
- 21.Tarentino A.L., Gomez C.M., Plummer T.H. Deglycosylation of asparagines-linked glycans by peptide: N-glycosidase F. Biochemistry. 1984;24:4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- 22.Johnson A., Smith R., Saxne T., Hickery M., Heinegard D. Fibronectin fragments cause release and degradation of collagen-binding molecule from equine explant cultures. Osteoarthritis Cartilage. 2004;12:149–159. doi: 10.1016/j.joca.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli U.K. Cleavage of structure proteins during the assembly of the head of bacteriophage-T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Towbin, H., Staehelin, T., Gordon, J. Electrophoretic transfer of proteins from poly-acrylamide gels to nitrocellulose sheets: procedure and some application. Proc. Natl. Acad. Sci. USA. 1979;76:4350–5354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson, J.J., Wells, G., Verhoeven, A.C., Felson, D.T. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum. 2000;43:22–29. doi: 10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]