Figure 3. Evaluation of the efficacy of HB-surface assembled CSNC prototypes.
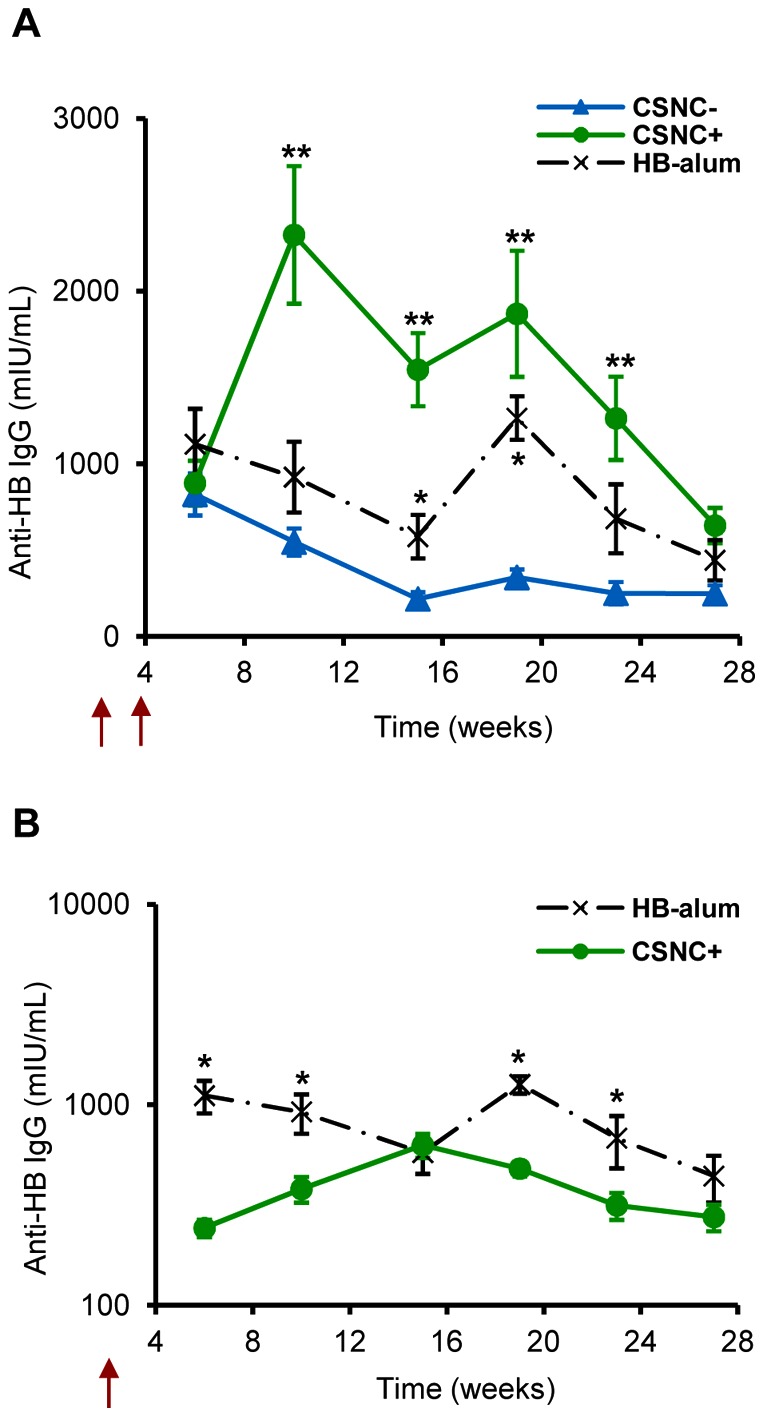
Immune response induced by the nanovaccine prototypes (CSNC− and CSNC+) and the positive control (HB-alum) administered intramuscularly following different vaccination protocols. (A) IgG anti-HB levels generated after prime-boost immunization (arrows, weeks 0–4) with both prototypes: CSNC− (blue –▴–); CSNC+ (green –•–) and HB-alum (black –·X·–) at the same dose (10 µg). Results are presented as mean ± SEM.*Alum or CSNC+ vs. CSNC− (p<0.05). ** CSNC+ vs. Alum and CSNC- (p<0.05). (B) IgG anti-HB (mIU/ml) levels elicited after a single dose (arrow) of CSNC+ (10 µg; green –•–) compared to alum-HB administered twice (10 µg, weeks 0 and 4) (black –·X·–). Results are presented as mean ± SEM.*(p<0.05).
