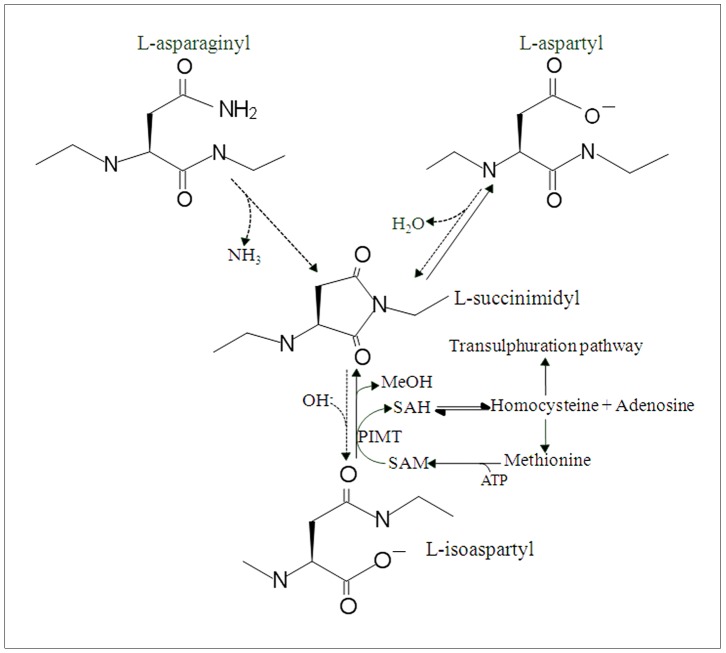
Figure 1. Isoaspartate protein damage formation and PIMT-mediated protein repair.
Within a peptide backbone, non-enzymatic deamidation of an asparagine-Xaa linkage or dehydration of an aspartic acid-Xaa linkage can give rise to a five-carbon succinimide (indicated by the dashed arrows). Succinimide hydrolysis (OH−) yields an isoaspartate residue (major product, indicated by the dashed arrow), or an aspartic acid (minor product, indicated by the right solid arrow). PIMT utilises SAM to carboxyl-methylate the isoaspartate forming a methyl ester that readily hydrolyses under physiological conditions to liberate methanol and reform the five-carbon succinimide (lower solid arrow). Subsequent succinimide hydrolysis will again yield isoaspartate or ‘repaired’ aspartate products (right solid arrow). PIMT activity is influenced by the levels of SAM and SAH, which are likewise governed by the enzyme and metabolite levels of the methionine metabolic pathway.