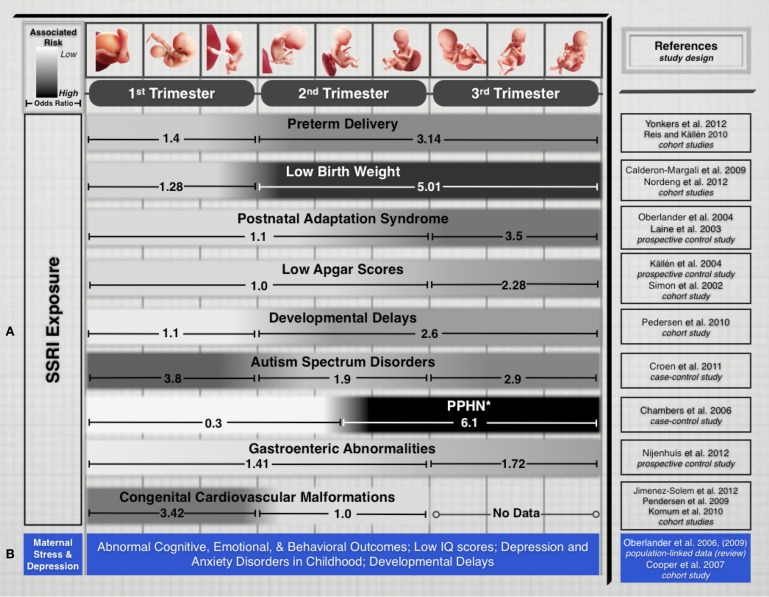
Figure 1.
(A) Treatment of maternal depression with SSRIs is associated with varying pregnancy outcomes. While every gestational stage of SSRI exposure has been implicated in increased risks for cognitive, physiological, or developmental teratogenicity, the period of exposure is an important factor that appears to influence clinical outcomes in the offspring. We limited this list to outcomes that have been the focus of several epidemiological studies in recent years and for which differential exposure data during pregnancy was available. (B) Untreated maternal depression and stress have been associated with several risks that affect cognitive and developmental outcomes. While associations are not generally correlated to specific trimesters, exposure to untreated maternal depression or stress during pregnancy pose adverse risks to fetal health and development. Study Selection and Data Extraction Studies were selected if they had clearly identified maternal SSRI exposure for specific trimesters of pregnancy and assessed neonatal outcomes. Epidemiological studies that included medium-to-large number samples exposed to different SSRI drugs were selected. Direct comparison of absolute odds ratio values across these studies is not possible due to varying specific study designs, adjustments for level of maternal depression and various sociodemographic and lifestyle factors, drug dosages, length of exposure, and SSRI treatment options. *PPHN, Persistent pulmonary hypertension of the newborn.