Abstract
Giardiasis is one of the most common causes of diarrheal disease worldwide. Treatment is primarily with 5-nitro antimicrobials, particularly metronidazole. Resistance to metronidazole has been described, and treatment failures can occur in up to 20% of cases, making development of alternative antigiardials an important goal. To this end, we have screened a chemical library of 746 approved human drugs and 164 additional bioactive compounds for activity against Giardia lamblia. We identified 56 compounds that caused significant inhibition of G. lamblia growth and attachment. Of these, 15 were previously reported to have antigiardial activity, 20 were bioactive but not approved for human use, and 21 were drugs approved for human use for other indications. One notable compound of the last group was the antirheumatic drug auranofin. Further testing revealed that auranofin was active in the low (4 to 6)-micromolar range against a range of divergent G. lamblia isolates representing both human-pathogenic assemblages A and B. Most importantly, auranofin was active against multiple metronidazole-resistant strains. Mechanistically, auranofin blocked the activity of giardial thioredoxin oxidoreductase, a critical enzyme involved in maintaining normal protein function and combating oxidative damage, suggesting that this inhibition contributes to the antigiardial activity. Furthermore, auranofin was efficacious in vivo, as it eradicated infection with different G. lamblia isolates in different rodent models. These results indicate that the approved human drug auranofin could be developed as a novel agent in the armamentarium of antigiardial drugs, particularly against metronidazole-resistant strains.
INTRODUCTION
Giardiasis is one of the most common human parasitic infections of the intestinal tract worldwide, affecting hundreds of millions of people, mostly in developing countries. It has been included in the Neglected Diseases Initiative of the WHO (1). Giardia exists in two forms, the infectious cyst and the disease-causing trophozoite that colonizes the small intestinal lumen. Cysts are spread through drinking water, food, and person-to-person contact. The clinical symptoms of giardiasis include diarrhea, abdominal pain, malabsorption, and weight loss. A recent cohort study following a major giardiasis outbreak in Norway showed that infection with Giardia lamblia was associated with a high prevalence of irritable bowel syndrome and chronic fatigue 3 years after acute illness (2), highlighting the major health impact of giardiasis even in areas where it is not endemic. Treatment of giardiasis relies on antimicrobial drug therapy, most commonly with 5-nitroheterocyclic drugs, particularly metronidazole and, more recently, nitazoxanide (3). However, cross-resistance among 5-nitro antimicrobials exists, and treatment failures occur in up to 20% of cases (4–6). Alternative antimicrobials exist, but these are generally less effective than 5-nitro drugs (3, 7).
One important strategy in the development of new antimicrobials is the screening of existing compound libraries for activities against particular target microbes. This strategy was successfully applied to several protozoan parasites, including G. lamblia and the enteric parasite Entamoeba histolytica (8–10). In particular, screening libraries of FDA-approved compounds has the obvious advantage that extensive preclinical testing is not required, thus accelerating the progression to clinical efficacy trials. In the current study, we have applied this strategy to a novel compound library with close to 750 approved human drugs to identify alternative antigiardial agents with promising new in vitro and in vivo activities. We show here that one of these compounds is the antirheumatic drug auranofin, indicating that this compound has great potential as a novel agent in the armamentarium of antigiardial drugs.
MATERIALS AND METHODS
Materials.
The chemical library screened in these studies was donated by Iconix Biosciences, Inc. (Foster City, CA), and consisted of 1,083 known bioactive compounds. Only 910 of these compounds were soluble at 20 mM in dimethyl sulfoxide (DMSO) and were subsequently used for activity screens. Of the 910 compounds, 603 are FDA-approved drugs (comprising about 40% of all ∼1,500 currently FDA-approved drugs), 143 are approved as human drugs in other countries (primarily Japan and different European nations), and 164 are bioactive but not approved human drugs. Thus, 746 compounds (or 82% of all tested compounds) are drugs approved for human use. Of the nonapproved compounds, 12 are generally recognized as safe (GRAS) natural products or food additives, and an additional 14 are veterinary drugs that are approved in different countries, while the remaining compounds are not approved for use in humans or animals. Information on indications and usage for FDA-approved drugs was obtained from the FDA website (Drugs@FDA [http://www.accessdata.fda.gov]). Auranofin was purchased from Enzo and dissolved in ethanol at 4 mg/ml, metronidazole was dissolved in DMSO, and diphenyleneiodonium chloride was purchased from Sigma and dissolved in DMSO at 10 mg/ml.
G. lamblia isolates and culture.
The following G. lamblia assemblage A isolates were used: WB (ATCC 50803); BRIS/83/HEPU/106 (106) (11), BRIS/83/HEPU/713 (713) (12), and their respective isogenic metronidazole-resistant lines, 713-M3 and 106-2ID10 (11, 12); C17-resistant cell line 106-17A, a derivative of 106 that had acquired metronidazole resistance after chronic in vitro exposure to the 5-nitroimidazole (5-NI) compound C17 (13); and WB-M1 and WB-M2, both of which are metronidazole-resistant lines generated in vitro from WB (4). In addition, the following assemblage B isolates of G. lamblia were employed: BRIS/91/HEPU/1279 (1279) (14) and 1279-M1, a metronidazole-resistant line of 1279 that was newly generated in the laboratory for these studies following the general strategies we used before for WB-M1 and WB-M2 (4).
All cell lines were grown in TYI-S-33 medium supplemented with 10% bovine calf serum and 1 mg/ml bovine bile (Giardia growth medium) under anaerobic conditions (AnaeroPack system; Remel). The metronidazole-resistant lines were routinely maintained in 50 μM metronidazole but were grown without the drug for 2 to 3 days before experiments.
Drug susceptibility assays and screening.
The chemical library was screened in 48-h growth assays in 96-well plates with automated image analysis for detection of attached cells as described before (9). Compounds were tested at 10 μM in the screens, and those that inhibited growth or attachment by >80% were considered active. Follow-up tests for individual compounds were done with a similar 48-h growth assay but with ATP determination as the readout for cell growth and survival (15). The 50% effective concentration (EC50), i.e., the drug concentration that inhibits G. lamblia growth by 50% compared to that of parallel cultures without drugs added, was determined by graphic extrapolation of the concentration-response curves using a nonlinear regression analysis of the dose-response curves (GraphPad Prism). Antigiardial potency is expressed as pEC50, i.e., the negative log10 value of the EC50.
Animal models of G. lamblia infection.
Adult C57BL/6 mice were obtained from The Jackson Laboratory and bred at UCSD. For infection of suckling mice (5 to 7 days old), G. lamblia trophozoites were grown to mid-logarithmic phase and administered by oral gavage at 107/mouse in a 50-μl volume in Giardia growth medium. After 2 days, mice were treated with auranofin by oral gavage once daily for 5 days. For trophozoite enumeration, the entire small intestine was removed, opened longitudinally, placed into 2 to 5 ml phosphate-buffered saline (PBS), and cooled on ice for 10 min. Live trophozoites were counted in a hemocytometer. Adult (10-week-old) Mongolian gerbils (Meriones unguiculatus) were obtained from Charles River Laboratories. Gerbils were fasted for 3 h and inoculated with 107 trophozoites in 200 μl of Giardia growth medium, and drug administrations and trophozoite enumerations were performed as described for mice. For assessment of drug toxicity in vivo, adult C57BL/6 mice were given five daily doses of 10 mg/kg auranofin. Small intestine, colon, liver, spleen, and kidneys were collected and fixed, and paraffin sections were prepared and stained with hematoxylin and eosin. Determinations of plasma levels of alkaline phosphatase, alanine aminotransferase (ALT), aspartate transaminase (AST), albumin, and bilirubin were performed by standard methods in the UCSD Murine Hematology and Coagulation Core Laboratory. All animal studies were reviewed and approved by the UCSD Institutional Animal Care and Use Committee.
Morphological Giardia studies.
To evaluate drug impact on trophozoite morphology, WB was plated overnight on glass-bottom plates (no. 1.5 cover glass; MatTek) and exposed to different concentrations of the test compounds for 3 h at 37°C. To minimize exposure to environmental oxygen in these short-term experiments, the cultures were placed into an anaerobic jar in which air was rapidly exchanged to 100% nitrogen by three 30-s cycles of gas removal and refilling with pure nitrogen. After incubation, live trophozoites were imaged by differential interference contrast microscopy (Perkin-Elmer UltraView Vox spinning-disk confocal microscope with a Hamamatsu EMCCD 1 k by 1 k camera) at × 60 magnification and 1- to 5-ms exposures.
Enzyme assays.
Recombinant Giardia thioredoxin reductase (GlTrxR) (XP_001707168) was amplified from DNA of G. lamblia 106, purified, cloned, and expressed in arabinose-inducible Escherichia coli BL21-AI as described previously (16). TrxR activity was assayed in 100 mM potassium phosphate buffer (pH 6.25) containing 150 nM GlTrxR and 200 μM NADPH by following the reduction of 1 mM 5,5′-dithiobis(2-nitrobenzoate) (DTNB) at λ = 412 nm (Δε = 13.6 mM−1 cm−1) (16). Half-maximal inhibitory concentrations (IC50s) for GlTrxR activity were calculated using Grafit 7 software (Erithacus Software).
Statistical analysis.
Trophozoite counts from infected animals were log10 transformed, and means and standard deviations (SD) were calculated from the log values. Samples without detectable trophozoites were assigned a log value equivalent to half of the detection limit of the assay (103 trophozoites/intestine). Differences between groups were compared by Wilcoxon rank sum test, with a P value of <0.05 considered significant.
RESULTS
Screening of chemical library against Giardia.
The chemical library tested in these studies contained 910 bioactive compounds, consisting mostly (82% of compounds) of approved human drugs and a smaller set of other bioactive molecules. This library had been used successfully for another parasite (8), which prompted us to evaluate it independently for activity against Giardia. Testing against G. lamblia strain WB by 48-h culture and image analysis of surviving cells revealed 56 compounds that caused significant inhibition of G. lamblia growth and attachment when tested at 10 μM. Of these, 15 had previously been reported to have antigiardial activity, including albendazole, furazolidone, tinidazole, genistein, and puromycin (Table 1), confirming the efficacy of our screen. Furthermore, 20 compounds are not drugs approved by the FDA or other national agencies for human use (Table 1). For example, cycloheximide is a general protein synthesis inhibitor, and thimerosal is a chemical preservative. Neither the compounds with known antigiardial activity nor the compounds not approved for human use were further pursued in these studies.
Table 1.
Antigiardial compounds with previously known activity and/or not approved for human use
| Compound | FDA approved (yr) | Known antigiardial | Reference |
|---|---|---|---|
| Albendazole | Yes (1996) | Yes | 3 |
| Disulfiram | Yes (1951) | Yes | 31 |
| Docetaxel | Yes (1996) | Yes | 8 |
| Furazolidone | Yes (1961) | Yes | 3 |
| Thiabendazole | Yes (1967) | Yes | 32 |
| Tinidazole | Yes (2004) | Yes | 3 |
| Vincristine | Yes (1963) | Yes | 33 |
| Aminacrine | No | Yes | 3 |
| Emetine | No | Yes | 34 |
| Fenbendazole | No | Yes | 35 |
| Genistein | No | Yes | 36 |
| Nocodazole | No | Yes | 35 |
| Oxfendazole | No | Yes | 35 |
| Puromycin | No | Yes | 37 |
| Secnidazole | No | Yes | 3 |
| Astemizole | No | No | |
| Bazedoxifene | No | No | |
| Brilliant green | No | No | |
| Cetylpyridinium bromide | No | No | |
| Chloroquinaldol | No | No | |
| Cycloeximide | No | No | |
| Diethylaminoethoxyhexestrol | No | No | |
| Ebastine | No | No | |
| Harringtonine | No | No | |
| Metergoline | No | No | |
| Perhexiline | No | No | |
| Piclamilast | No | No | |
| Podophyllotoxin | No | No | |
| Roxarsone | No | No | |
| Sch-351591 | No | No | |
| Sporidesmin A | No | No | |
| Terfenadine | No | No | |
| Thimerosal | No | No | |
| Thiram | No | No | |
| Vindesine | No | No |
The remaining 21 compounds approved for human use with antigiardial activity in the initial screens could be divided into four functional groups (Table 2): (i) antineoplastic agents (n = 5), (ii) adrenergic antagonists (n = 4), (iii) antimicrobials (n = 7), and (iv) compounds with miscellaneous activities and indications (n = 5). Retesting of representative compounds of groups 1 and 2 confirmed their activity against Giardia, with the exception of imatinib (Fig. 1). Because antineoplastic agents (group 1) can have serious side effects, these compounds will probably not be developed for treating giardiasis. The antigiardial activity of different adrenergic antagonists (group 2) extends a prior report of such activity for the β-adrenergic antagonist propranolol (17), but the ultimate utility of these agents in the treatment of giardiasis is also questionable due to their intended effects in the cardiovascular system and/or central nervous system. The known antimicrobials (group 3) were not further evaluated in this study. Of the remaining miscellaneous compounds (group 4), several have endocrine and hormonal effects (e.g., cisapride and clomiphene), also compromising their possible use for giardiasis treatment. However, of particular interest in this group was auranofin, which has been used for over 25 years in the treatment of rheumatoid arthritis and has more recently been shown to be active against several other parasites and bacteria (10, 18, 19). Therefore, we decided to explore in detail the activity of auranofin against Giardia.
Table 2.
Newly identified FDA-approved drugs and other safe compounds with antigiardial activitya
| Drug type | Compound | Yr of FDA approval | Indication(s)/activity(ies) (reference) |
|---|---|---|---|
| Antineoplastic | Cladribine | 1993 | Hairy cell leukemia |
| agents | Idarubicin | 1990 | Acute myeloid leukemia |
| Imatinib | 2003 | Chronic myeloid leukemia, gastrointestinal stromal cell tumors | |
| Mitoxantrone | 1987 | Acute myeloid leukemia, prostate cancer, multiple sclerosis | |
| Vinorelbine | 1994 | Lung cancer | |
| Adrenergic antagonists | Carvedilol | 1995 | Chronic heart failure, hypertension |
| Doxazosin | 1990 | Hypertension, benign prostate hyperplasia | |
| Prazosin | 1976 | Hypertension | |
| Thioridazineb | 1962 | Psychosis | |
| Antimicrobials | Chlorhexidine | Topical antiseptic (38) | |
| Clioquinol | Antiprotozoal, antifungal (39) | ||
| Gramicidin | Topical antibiotic (40) | ||
| Pyrithione zinc | Seborrhoeic dermatitis (41) | ||
| Rifabutin | 1992 | Mycobacterium infection | |
| Tannic acid | Food additive (42) | ||
| Vidarabineb | 1976 | Ocular herpesvirus infection | |
| Other | Auranofin | 1985 | Rheumatoid arthritis |
| Cisapride | 1997 | Gastroesophageal reflux disease | |
| Clomiphene | 1967 | Ovulatory dysfunction | |
| Loperamide | 1976 | Diarrhea | |
| Roflumilast | 2011 | Chronic obstructive pulmonary disease |
Compounds without an FDA approval date are not FDA-approved drugs or were withdrawn from their original indications but are used in clinical practice in combination therapies or other applications (as discussed in the listed references).
Discontinued.
Fig 1.

Retesting of selected antigiardial compounds from a chemical library. The indicated compounds were tested quantitatively for activity (pEC50) against G. lamblia WB in a 48-h growth assay using ATP measurements as a readout. Data are shown as mean + SD (n = 3). The dashed horizontal line represents the highest tested drug concentration.
Antigiardial activity of auranofin in vitro.
Auranofin inhibited growth and survival of several different G. lamblia isolates, belonging to both assemblages A and B, with half-maximal effective concentrations (EC50s) of 4 to 6 μM when tested in a 48-h assay (Fig. 2A). This activity falls within the low-micromolar range of plasma auranofin levels after standard doses in humans (20), suggesting that this drug may be therapeutic against giardiasis in vivo, particularly since the drug is given orally and giardial infection is limited to the small intestinal lumen. Importantly, auranofin was able to overcome resistance to the most commonly used antigiardial drug, metronidazole. Thus, the EC50 for auranofin was not significantly different between metronidazole-sensitive parental Giardia isolates and several of their metronidazole-resistant isogenic derivative lines, whereas the EC50 for metronidazole was 8- to 40-fold higher in the resistant lines than in the parental lines (Fig. 2B). The drug-resistant lines represent a spectrum of different resistance mechanisms (15) and were independently derived from geographically diverse G. lamblia isolates, comprising both assemblage A isolates (WB, 713, and 106) and a newly generated metronidazole-resistant line of the assemblage B isolate 1279. These in vitro studies indicate that auranofin is broadly active against diverse G. lamblia isolates and completely overrides resistance to the most widely used antigiardial drug, metronidazole.
Fig 2.
Activity of auranofin against divergent metronidazole-sensitive and -resistant G. lamblia isolates. Metronidazole (MZ) and auranofin (AF) were tested for activity (pEC50) against the depicted G. lamblia lines. WB-M1 and WB-M2 are metronidazole-resistant derivatives of the parental WB line, 713-M3 is a metronidazole-resistant derivative of the parental 713 line, 2ID10 and 106-C17 are metronidazole-resistant and C17-resistant (C17 is a 5-nitroimidazole related to metronidazole) lines, respectively, of the parental 106 isolate, and 1279-MR is a metronidazole-resistant derivative of the parental 1279 isolate. WB, 713, and 106 belong to assemblage A, while 1279 belongs to assemblage B. (A and B) Representative examples of the concentration-response curves for the effects of AF (A) and MZ (B) on Giardia survival and growth, relative to maximal growth (as determined by total ATP levels) in solvent-treated control cultures. (C and D) Summary of data from several independent experiments for AF (C) and MZ (D). All data are shown as mean ± standard error of the mean (SEM) for panels A and B (one representative experiment) and mean + SD (n = 3 experiments) for panels C and D, *, P < 0.05 versus the respective parental isolates.
Auranofin inhibits TrxR in G. lamblia.
Auranofin inactivates the flavoenzyme thioredoxin-glutathione reductase in several parasites, including Schistosoma mansoni and Haemonchus contortus, which has been proposed to account for the antimicrobial activity of auranofin (18, 21). Giardia possesses a thioredoxin oxidoreductase (TrxR) with marked homology to the enzyme in other microbes (16). We therefore tested whether auranofin can inhibit this enzyme in Giardia. Purified recombinant Giardia TrxR was incubated with increasing concentrations of auranofin and the substrate DTNB, which changes color upon enzymatic reduction by TrxR. Auranofin inhibited TrxR activity with an IC50 of 152 ± 12 nM (Fig. 3A). For comparison, this activity is ∼100-fold more potent than the inhibitory activity of auranofin for human thioredoxin reductase (IC50 of 18 μM) (22) but 15-fold less potent than in the helminth S. mansoni (IC50 of 10 nM) (18). These data indicate that auranofin is an effective inhibitor of Giardia TrxR.
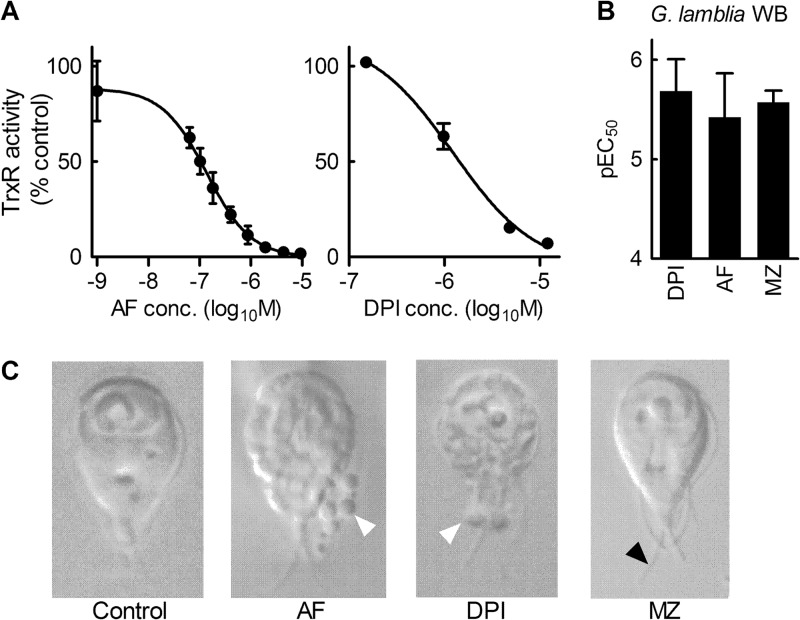
Fig 3.
Inhibition of G. lamblia TrxR by auranofin. (A) In vitro activity of purified, recombinant TrxR from G. lamblia was determined with the substrate DTNB in the presence of increasing concentrations of auranofin (AF) or DPI. Data are expressed relative to the control activity without drugs and are shown as mean ± SD (n = 3). (B) The antigiardial activities of DPI, AF, and metronidazole (MZ) were determined by 48-h growth assay and are shown as pEC50 (mean + SD). (C) The effects of the indicated drugs on the morphology of G. lamblia WB were determined with the aid of a spinning-disk confocal microscope. MZ-treated cells exhibited marked slowing of flagellar beating, since flagella could be easily captured by the camera (black arrowhead), whereas the flagella in untreated cells could barely be detected under these conditions due to their rapid movement. By comparison, AF and DPI treatment caused only modest flagellar slowing but instead led to marked cellular blebbing (white arrowheads).
To determine whether TrxR inhibition by auranofin may account for its antigiardial effects, we tested a broad-spectrum flavoenzyme inhibitor, diphenyleneiodonium (DPI) (23). Giardia TrxR was effectively inhibited by DPI with an IC50 of 1.3 ± 0.2 μM (Fig. 3A). Furthermore, DPI had marked antigiardial activity, with IC50s similar to those of auranofin and metronidazole (Fig. 3B). Morphological analysis of cell death caused by the three agents auranofin, DPI, and metronidazole showed similarities between DPI and auranofin but not metronidazole. Thus, we observed by spinning-disk phase-contrast microscopy that auranofin-treated cells had an irregular shape with numerous blebs extending from the cell membrane (Fig. 3C) and modest slowing of flagellar beating. Similarly, DPI-treated cells displayed irregular membrane structures and blebbing (Fig. 3C), as well as flagellar slowing. In contrast, cells treated with metronidazole showed a different phenotype, with little apparent change in overall cell morphology but almost complete absence of flagellar movement (Fig. 3C). Thus, both auranofin and the flavoenzyme enzyme inhibitor DPI inhibit Giardia TrxR and cause similar morphological forms of cell death, while metronidazole leads to a different cell death morphology. Together with the observation that auranofin overcomes metronidazole resistance (Fig. 2A), these data indicate that auranofin and DPI appear to have similar mechanisms of antimicrobial action in Giardia, while metronidazole acts by a different mechanism. These findings suggest that inhibition of TrxR may be responsible, at least in part, for the antigiardial activity of auranofin.
In vivo activity of auranofin against giardiasis.
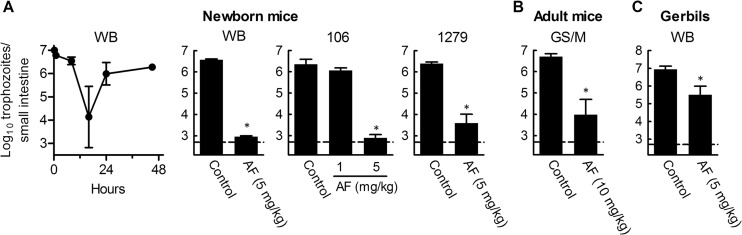
As an important next preclinical step in assessing the potential of auranofin for treating giardiasis in humans, we evaluated its activity in rodent models of giardiasis. Newborn mice (5 to 7 days old) were infected orally with 107 trophozoites of two assemblage A isolates, 106 and WB, or the assemblage B isolate 1279, and infectious load was assessed by enumerating live trophozoites in the small intestine. Initial time course studies showed that trophozoite numbers declined rapidly to ∼104 in the first 16 h after inoculation but then recovered after 1 to 2 days to reach steady-state levels of 106 to 107 (Fig. 4A) for the next 5 to 7 days, indicating that the parasites were actively proliferating at that time. Consequently, to assess drug efficacy in an established infection, we started auranofin treatment after 2 days and continued once daily for 5 days. Auranofin was highly effective against G. lamblia WB and 106 and slightly less so against G. lamblia 1279 at a dose of 5 mg/kg (Fig. 4A). By comparison, at a lower dose of 1 mg/kg, auranofin treatment resulted in only a modest 2-fold decrease in trophozoite numbers for the 106 isolate (Fig. 4A). Furthermore, adult mice infected with another assemblage B isolate, GS/M, were also effectively treated with auranofin (Fig. 4B). To extend these findings to another host species, we infected adult gerbils with WB trophozoites and, beginning on day 7, treated them daily for 5 days with 5 mg/kg auranofin. Treatment significantly decreased trophozoite numbers by ∼25-fold (Fig. 4C). These results demonstrate that auranofin is efficacious against infection with a range of G. lamblia isolates in different rodent models. None of the rodents treated with auranofin showed adverse effects, as determined by body weight or general appearance. Furthermore, adult mice treated with five daily doses of 10 mg/kg auranofin showed no significant abnormalities upon histological analysis of liver, kidneys, spleen, or intestinal tract or when analyzed for plasma levels of liver enzymes (alkaline phosphatase, AST, and ALT), albumin, or bilirubin, suggesting that auranofin was safe at an effective dose.
Fig 4.
In vivo efficacy of auranofin in rodent models of giardiasis. Suckling mice (5 to 7 days old), adult mice, or adult gerbils were infected with the indicated G. lamblia isolates. After 2 days (mice) or 7 days (gerbils), animals were given one daily dose of auranofin (AF) for 5 days or were given solvent alone (2.5 to 25% ethanol in PBS) as a control. Afterwards, live trophozoites in the small intestine were enumerated. Data are mean + SD for 3 to 6 mice or 4 gerbils per group; P < 0.05 by rank sum test versus PBS-treated controls. The dashed horizontal line represents the detection limit of the assay.
DISCUSSION
These studies have utilized a chemical library screening strategy to identify the gold-containing antirheumatic drug auranofin (brand name, Ridaura) as a promising new agent for the treatment of giardiasis. Gold preparations have been used since ancient times for various ailments, but their controlled use for specific diseases began only in the first few decades of the 20th century. Injectable gold compounds were found to be active against arthritic diseases starting in the 1930s, while the oral formulation, auranofin, was developed much later and shown to be effective in rheumatoid arthritis in the 1970s (24). Approved by the FDA in 1985, auranofin has been used in the treatment of rheumatoid arthritis, especially in patients who have failed other therapies, as well as for pemphigus and psoriatic arthritis (25). Its use has declined in recent years due to improved efficacy of newer antirheumatics and the significant adverse effects of auranofin during long-term treatment. In particular, diarrhea and other gastrointestinal adverse effects are fairly common, although rarely life-threatening, after standard doses of oral auranofin over months (25). However, an antimicrobial course would be much shorter than the long-term treatment of rheumatoid arthritis, and therefore side effects may be mitigated. Now off patent, auranofin is relatively inexpensive, but it is currently rarely employed for arthritic indications. However, it has gained new interest with the recent discoveries that it has significant antiparasitic activity against S. mansoni (18), Trypanosoma brucei (26), E. histolytica (10), and now Giardia, as shown here. Because the pharmacokinetics and toxicology of this FDA-approved compound are well understood (25), these new potential indications could be rapidly evaluated in clinical trials. Our observations that it is active in vitro against diverse Giardia strains at low-micromolar concentrations, which are within the range of the plasma levels after standard oral doses (25), and efficacious in two animal models of giardiasis suggest that auranofin has great potential to be a valuable addition to the existing repertoire of antigiardial drugs.
Auranofin inhibited the activity of G. lamblia TrxR at submicromolar concentrations in vitro. This enzyme maintains the dithiol-containing small redox protein thioredoxin in a reduced state. Because Giardia lacks the glutathione found in most other eukaryotic cells, thioredoxin is the major cellular redox control system that keeps protein thiols in their normally reduced state in the parasite, which keeps key proteins functionally active. In addition, thioredoxin is important for combating oxidative stress that can result from exposure of trophozoites to oxygen in the small intestinal lumen (27). The importance of TrxR/thioredoxin-dependent detoxification is enhanced by the absence in Giardia of conventional enzymes, such as superoxide dismutase, catalase, and peroxidase, that detoxify reactive oxygen species in most other organisms (27). Blockade of these critical TrxR actions by auranofin would predictably be detrimental to Giardia trophozoite survival, similar to what has been shown for Schistosoma (18, 28) and proposed for Entamoeba (10). Our functional and morphological data with DPI, an inhibitor of flavin-dependent redox processes including TrxR/thioredoxin (23), support this notion. However, despite the compelling case for a role of TrxR in mediating auranofin actions in Giardia, definitive proof for such a role is currently lacking and may be difficult to establish. Knockdown of TrxR has not been reported in Giardia, and might be hard to achieve given the relatively high abundance of the protein (16). Furthermore, if TrxR has the postulated critical cell functions, stable knockdown would not be compatible with survival, precluding evaluation of auranofin in cells lacking TrxR. Indirect evidence that TrxR activity is inhibited in auranofin-treated live cells could confirm our in vitro observation, but we have so far not been able to establish a suitable functional assay in Giardia. Even if such data could be obtained, they would not address the question whether TrxR is the only relevant target for auranofin. Nonetheless, taken together, our results suggest that TrxR inhibition is likely to contribute to the antigiardial activity of auranofin.
Auranofin was equally active against divergent Giardia strains with different forms of resistance against the most commonly used antigiardial drug, metronidazole, suggesting that auranofin could be a treatment alternative if patients fail standard metronidazole therapy (5). This alternative would be a particularly valuable therapeutic option, because metronidazole-resistant Giardia exhibits cross-resistance with all other clinically used 5-nitro antimicrobials (4). Moreover, the ability of auranofin to override metronidazole resistance suggests that this drug has a mechanism of action different from that of metronidazole. Interestingly, attenuated flavin metabolism, which is required for TrxR activity, has been implicated as one pathway of metronidazole resistance, yet TrxR expression was not decreased under these conditions (16). If TrxR is the key target of auranofin, this finding could explain why auranofin activity is not compromised by metronidazole resistance in this situation. It also underlines that TrxR may be a promising target for future development of drugs against giardiasis.
The strategy of screening existing drugs for potential new activities, as done here and in other studies (10, 29, 30), can accelerate the process of advancing drugs from preclinical testing to clinical trials and ultimate clinical use. Furthermore, this strategy is cost-effective if the screened libraries consist of approved human drugs, as it can minimize the considerable costs and uncertainties of preclinical safety testing. Reducing development times and costs is always important in the development of any new drug, but this is especially so for new drugs against “neglected” diseases such as giardiasis (1). This drug-recycling strategy is not a panacea to address all needs for the development of new drugs against infectious diseases, yet it provides a welcome respite in the battle against pathogens and their tenacious tendency to adapt to existing antimicrobials by developing drug resistance. In the case of giardiasis, what is needed next is a clinical trial to validate the clinical efficacy of auranofin and determine the minimal course of treatment. The optimal treatment dose would also have to be established, particularly given the slight difference in efficacy we observed at the same dose between mice and gerbils, but the starting point would presumably be the auranofin doses that have proven efficacious in rheumatoid arthritis (25).
ACKNOWLEDGMENTS
This work was supported by NIH U01 Cooperative Research Agreements AI75527 and AI77822, NIH grant DK35108, and the UCSD Digestive Diseases Research Development Center (DK80506).
Compounds for the screened library were kindly provided by Michelle R. Arkin and Steven Chen of the UCSF Small Molecule Discovery Center. We thank Elaine Hanson and Lucia Hall for technical assistance.
Footnotes
Published ahead of print 12 February 2013
REFERENCES
- 1. Savioli L, Smith H, Thompson A. 2006. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol. 22:203–208 [DOI] [PubMed] [Google Scholar]
- 2. Wensaas KA, Langeland N, Hanevik K, Morch K, Eide GE, Rortveit G. 2012. Irritable bowel syndrome and chronic fatigue 3 years after acute giardiasis: historic cohort study. Gut 61:214–219 [DOI] [PubMed] [Google Scholar]
- 3. Gardner TB, Hill DR. 2001. Treatment of giardiasis. Clin. Microbiol. Rev. 14:114–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tejman-Yarden N, Millman M, Lauwaet T, Davids BJ, Gillin FD, Dunn L, Upcroft JA, Miyamoto Y, Eckmann L. 2011. Impaired parasite attachment as fitness cost of metronidazole resistance in Giardia lamblia. Antimicrob. Agents Chemother. 55:4643–4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Escobedo AA, Cimerman S. 2007. Giardiasis: a pharmacotherapy review. Expert Opin. Pharmacother 8:1885–1902 [DOI] [PubMed] [Google Scholar]
- 6. Upcroft P, Upcroft JA. 2001. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 14:150–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wright JM, Dunn LA, Upcroft P, Upcroft JA. 2003. Efficacy of antigiardial drugs. Expert Opin. Drug Saf. 2:529–541 [DOI] [PubMed] [Google Scholar]
- 8. Chen CZ, Kulakova L, Southall N, Marugan JJ, Galkin A, Austin CP, Herzberg O, Zheng W. 2011. High-throughput Giardia lamblia viability assay using bioluminescent ATP content measurements. Antimicrob. Agents Chemother. 55:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gut J, Ang KK, Legac J, Arkin MR, Rosenthal PJ, McKerrow JH. 2011. An image-based assay for high throughput screening of Giardia lamblia. J. Microbiol. Methods 84:398–405 [DOI] [PubMed] [Google Scholar]
- 10. Debnath A, Parsonage D, Andrade RM, He C, Cobo ER, Hirata K, Chen S, Garcia-Rivera G, Orozco E, Martinez MB, Gunatilleke SS, Barrios AM, Arkin MR, Poole LB, McKerrow JH, Reed SL. 2012. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat. Med. 18:956–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boreham PF, Upcroft J, Upcroft P, Andrews R. 1988. Zoonotic Giardia—the debate goes on. Parasitol. Today 4:322. [DOI] [PubMed] [Google Scholar]
- 12. Townson SM, Laqua H, Upcroft P, Boreham PF, Upcroft JA. 1992. Induction of metronidazole and furazolidone resistance in Giardia. Trans. R. Soc. Trop. Med. Hyg. 86:521–522 [DOI] [PubMed] [Google Scholar]
- 13. Dunn LA, Burgess AG, Krauer KG, Eckmann L, Vanelle P, Crozet MD, Gillin FD, Upcroft P, Upcroft JA. 2010. A new-generation 5-nitroimidazole can induce highly metronidazole-resistant Giardia lamblia in vitro. Int. J. Antimicrob. Agents 36:37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Upcroft JA, Boreham PF, Campbell RW, Shepherd RW, Upcroft P. 1995. Biological and genetic analysis of a longitudinal collection of Giardia samples derived from humans. Acta Trop. 60:35–46 [DOI] [PubMed] [Google Scholar]
- 15. Valdez CA, Tripp JC, Miyamoto Y, Kalisiak J, Hruz P, Andersen YS, Brown SE, Kangas K, Arzu LV, Davids BJ, Gillin FD, Upcroft JA, Upcroft P, Fokin VV, Smith DK, Sharpless KB, Eckmann L. 2009. Synthesis and electrochemistry of 2-ethenyl and 2-ethanyl derivatives of 5-nitroimidazole and antimicrobial activity against Giardia lamblia. J. Med. Chem. 52:4038–4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leitsch D, Burgess AG, Dunn LA, Krauer KG, Tan K, Duchene M, Upcroft P, Eckmann L, Upcroft JA. 2011. Pyruvate:ferredoxin oxidoreductase and thioredoxin reductase are involved in 5-nitroimidazole activation while flavin metabolism is linked to 5-nitroimidazole resistance in Giardia lamblia. J. Antimicrob. Chemother. 66:1756–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farthing MJ, Inge PM, Pearson RM. 1987. Effect of d-propranolol on growth and motility of flagellate protozoa. J. Antimicrob. Chemother. 20:519–522 [DOI] [PubMed] [Google Scholar]
- 18. Angelucci F, Sayed AA, Williams DL, Boumis G, Brunori M, Dimastrogiovanni D, Miele AE, Pauly F, Bellelli A. 2009. Inhibition of Schistosoma mansoni thioredoxin-glutathione reductase by auranofin: structural and kinetic aspects. J. Biol. Chem. 284:28977–28985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jackson-Rosario S, Cowart D, Myers A, Tarrien R, Levine RL, Scott RA, Self WT. 2009. Auranofin disrupts selenium metabolism in Clostridium difficile by forming a stable Au-Se adduct. J. Biol. Inorg Chem. 14:507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gottlieb NL. 1986. Pharmacology of auranofin: overview and update. Scand. J. Rheumatol. Suppl. 63:19–28 [PubMed] [Google Scholar]
- 21. Hudson AL, Sotirchos IM, Davey MW. 2010. Substrate specificity of the mitochondrial thioredoxin reductase of the parasitic nematode Haemonchus contortus. Parasitol. Res. 107:487–493 [DOI] [PubMed] [Google Scholar]
- 22. Gromer S, Arscott LD, Williams CH, Jr, Schirmer RH, Becker K. 1998. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem. 273:20096–20101 [DOI] [PubMed] [Google Scholar]
- 23. Leitsch D, Kolarich D, Duchene M. 2010. The flavin inhibitor diphenyleneiodonium renders Trichomonas vaginalis resistant to metronidazole, inhibits thioredoxin reductase and flavin reductase, and shuts off hydrogenosomal enzymatic pathways. Mol. Biochem. Parasitol. 171:17–24 [DOI] [PubMed] [Google Scholar]
- 24. Kean WF, Hart L, Buchanan WW. 1997. Auranofin. Br. J. Rheumatol. 36:560–572 [DOI] [PubMed] [Google Scholar]
- 25. Kean WF, Kean IR. 2008. Clinical pharmacology of gold. Inflammopharmacology 16:112–125 [DOI] [PubMed] [Google Scholar]
- 26. Lobanov AV, Gromer S, Salinas G, Gladyshev VN. 2006. Selenium metabolism in Trypanosoma: characterization of selenoproteomes and identification of a Kinetoplastida-specific selenoprotein. Nucleic Acids Res. 34:4012–4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown DM, Upcroft JA, Edwards MR, Upcroft P. 1998. Anaerobic bacterial metabolism in the ancient eukaryote Giardia duodenalis. Int. J. Parasitol. 28:149–164 [DOI] [PubMed] [Google Scholar]
- 28. Kuntz AN, Davioud-Charvet E, Sayed AA, Califf LL, Dessolin J, Arner ES, Williams DL. 2007. Thioredoxin glutathione reductase from Schistosoma mansoni: an essential parasite enzyme and a key drug target. PLoS Med. 4:e206 doi:10.1371/journal.pmed.0040206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chopra S, Torres-Ortiz M, Hokama L, Madrid P, Tanga M, Mortelmans K, Kodukula K, Galande AK. 2010. Repurposing FDA-approved drugs to combat drug-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 65:2598–2601 [DOI] [PubMed] [Google Scholar]
- 30. Chopra S, Matsuyama K, Hutson C, Madrid P. 2011. Identification of antimicrobial activity among FDA-approved drugs for combating Mycobacterium abscessus and Mycobacterium chelonae. J. Antimicrob. Chemother. 66:1533–1536 [DOI] [PubMed] [Google Scholar]
- 31. Nash T, Rice WG. 1998. Efficacies of zinc-finger-active drugs against Giardia lamblia. Antimicrob. Agents Chemother. 42:1488–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edlind TD, Hang TL, Chakraborty PR. 1990. Activity of the anthelmintic benzimidazoles against Giardia lamblia in vitro. J. Infect. Dis. 162:1408–1411 [DOI] [PubMed] [Google Scholar]
- 33. Sandhu H, Mahajan RC, Ganguly NK. 2004. Flowcytometric assessment of the effect of drugs on Giardia lamblia trophozoites in vitro. Mol. Cell. Biochem. 265:151–160 [DOI] [PubMed] [Google Scholar]
- 34. Alanis AD, Calzada F, Cedillo-Rivera R, Meckes M. 2003. Antiprotozoal activity of the constituents of Rubus coriifolius. Phytother. Res. 17:681–682 [DOI] [PubMed] [Google Scholar]
- 35. Morgan UM, Reynoldson JA, Thompson RC. 1993. Activities of several benzimidazoles and tubulin inhibitors against Giardia spp. in vitro. Antimicrob. Agents Chemother. 37:328–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khan IA, Avery MA, Burandt CL, Goins DK, Mikell JR, Nash TE, Azadegan A, Walker LA. 2000. Antigiardial activity of isoflavones from Dalbergia frutescens bark. J. Nat. Prod. 63:1414–1416 [DOI] [PubMed] [Google Scholar]
- 37. Singer SM, Yee J, Nash TE. 1998. Episomal and integrated maintenance of foreign DNA in Giardia lamblia. Mol. Biochem. Parasitol. 92:59–69 [DOI] [PubMed] [Google Scholar]
- 38. Lim KS, Kam PC. 2008. Chlorhexidine—pharmacology and clinical applications. Anaesthesia Intensive Care 36:502–512 [DOI] [PubMed] [Google Scholar]
- 39. Mao X, Schimmer AD. 2008. The toxicology of Clioquinol. Toxicol. Lett. 182:1–6 [DOI] [PubMed] [Google Scholar]
- 40. Mogi T, Kita K. 2009. Gramicidin S and polymyxins: the revival of cationic cyclic peptide antibiotics. Cell. Mol. Life Sci. 66:3821–3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guthery E, Seal LA, Anderson EL. 2005. Zinc pyrithione in alcohol-based products for skin antisepsis: persistence of antimicrobial effects. Am. J. Infect. Control 33:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chung KT, Wong TY, Wei CI, Huang YW, Lin Y. 1998. Tannins and human health: a review. Crit. Rev. Food Sci. Nutr. 38:421–464 [DOI] [PubMed] [Google Scholar]