Figure 1.
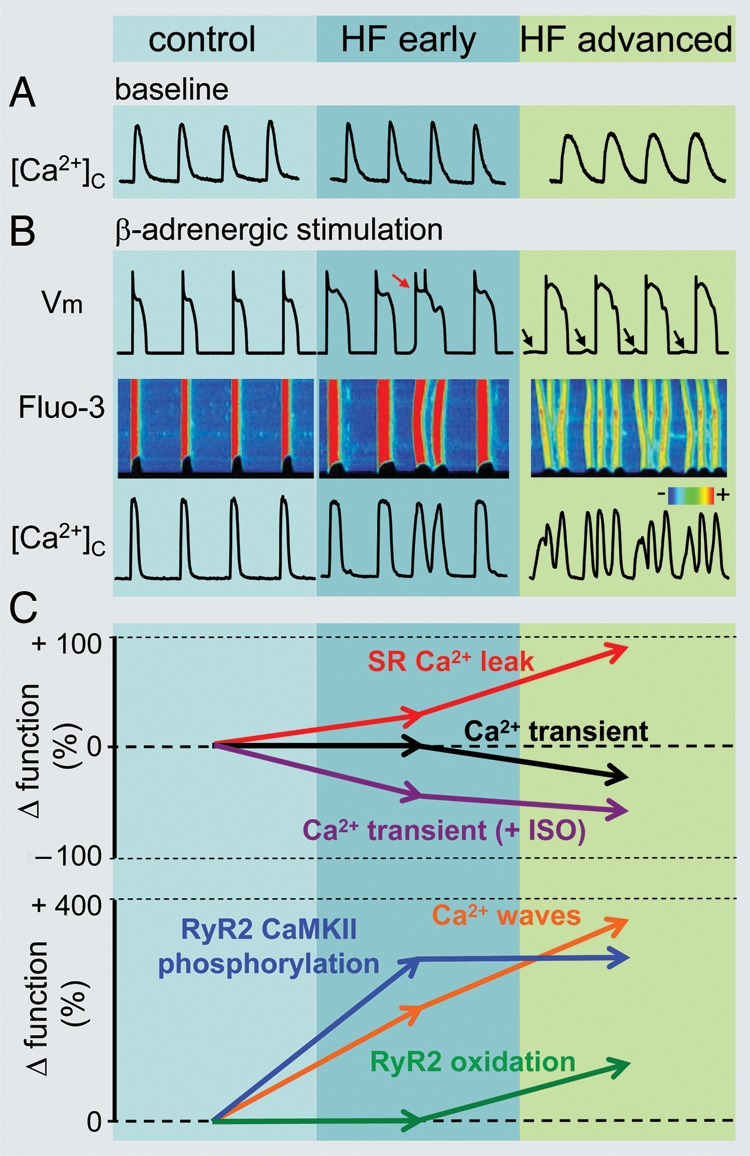
Remodelling of intracellular Ca2+cycling during heart failure (HF) progression. (A) Representative Ca2+ transients recorded in control as well as in ventricular myocytes isolated from hearts with early and advanced stages of HF. (B) Representative action potential (AP) traces (top) along with corresponding line scan images of Fluo-3 fluorescence (middle) and Ca2+ transients ([Ca2+]c; bottom) recorded during β-adrenergic receptor stimulation in the three different types of cardiomyocytes. Arrows indicate delayed after-depolarizations. Red arrow points to extrasystolic AP. (C) Summary graphs illustrate that progression of HF is associated with early and progressive increase in the rate of SR Ca2+ leak (red line). The amplitude of Ca2+ transients recorded under baseline conditions (black line) decreases with significant time-delay in respect to elevation of leak during the onset of HF. Such relationships are attributable to the capacity of myocyte Ca2+ handling for autoregulation until SR is depleted by a drastic increase in the SR Ca2+ leak (see text for detailed explanation). In contrast, the frequency of diastolic Ca2+ waves recorded in the presence of β-adrenergic agonist isoproterenol (ISO) (brown line) parallels increases in the SR Ca2+ leak. Ca2+ waves facilitate diastolic SR Ca2+ loss resulting in decrease in Ca2+ transient amplitude (+ISO; purple line). Note that in advanced stages of HF persistent cytosolic Ca2+ oscillations effectively uncouple electrical excitation from mechanical response. Progressive alterations in Ca2+ cycling in HF are coupled to sequential modification of RyR2 by CaMKII-dependent phosphorylation (blue line) and oxidation (green line). Adapted from Belevych et al.27
