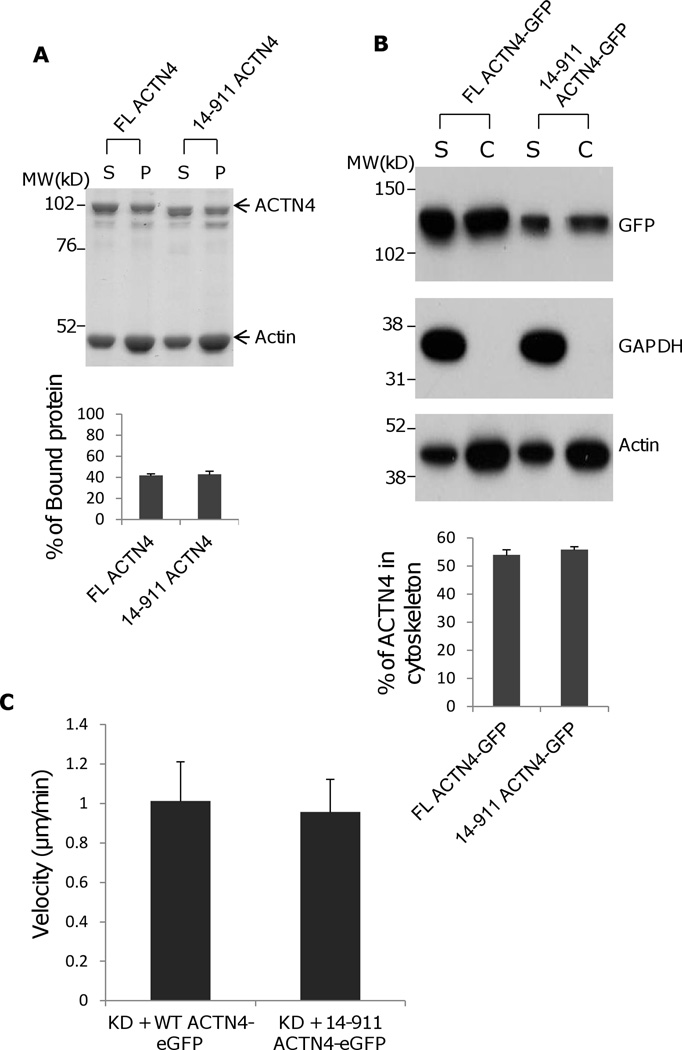
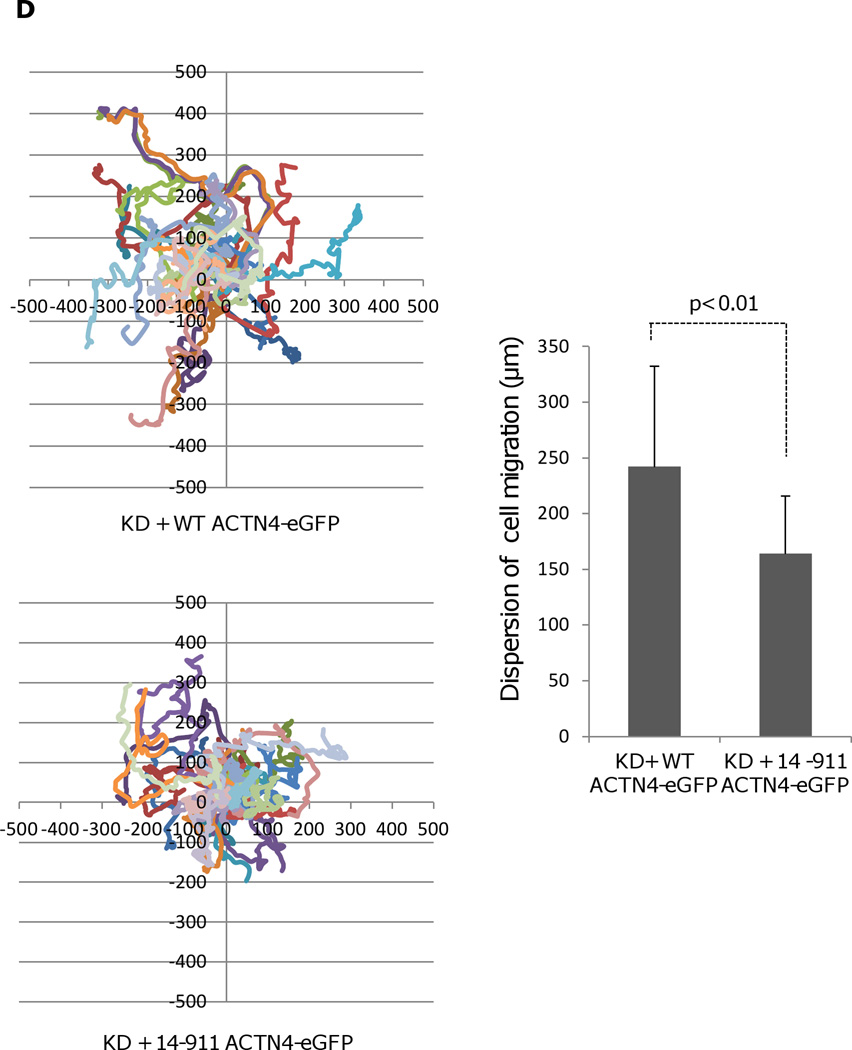
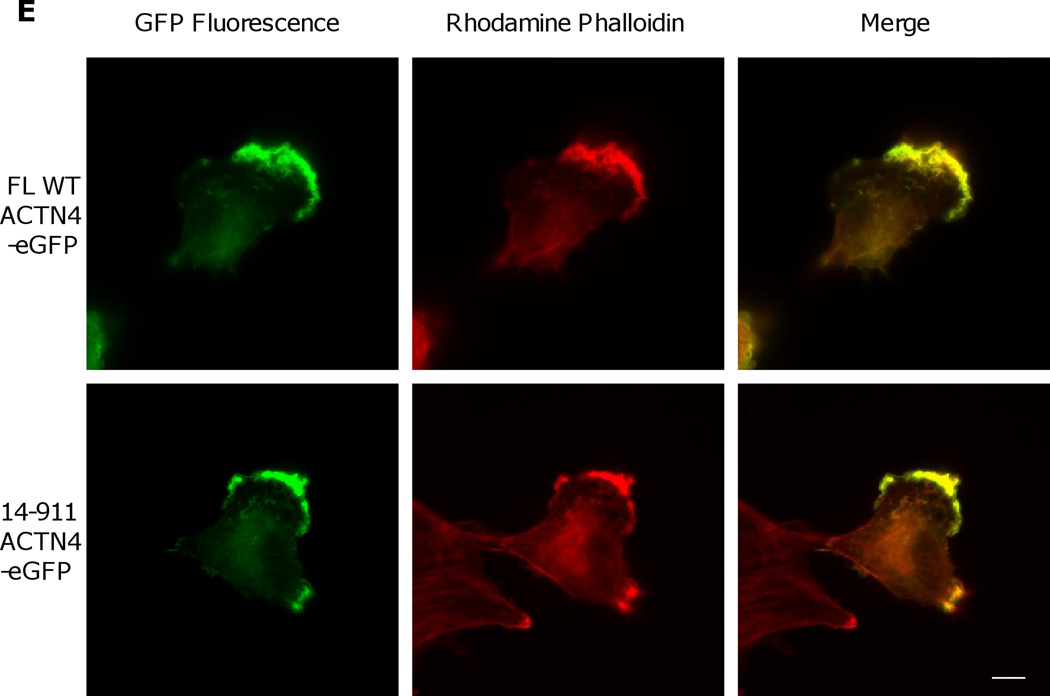
Fig. 2.
Actin binding activity of 14–911 and its effects on cell migration. (A)WT ACTN4 and its truncated fragment 14–911 expressed and purified in E.Coli and dialyzed completely against actin binding buffer were incubated with G-actin in actin binding buffer at room temperature for 1h. F-actin and bound ACTN4 were pulled down by centrifugation at 100,000 × g for 30 min at 25 °C. Equal amounts of supernatant and pellets were loaded and separated by polyacrylamide gel followed by Coomassie staining and quantified using Image J software and represented by graph. “S” stands for supernatant and “P” stands for pellet. Image analysis and quantitation of three independent experiments are shown. (B) Immunoblotting of soluble and cytoskeletal ACTN4. See methods for the isolation of cytoskeleton. Quantification presents the amount of ACTN4 in cytoskeleton. The densitometry results for the ACTN4-eGFP immunoblotting are shown. (C) Live cell tracking of NR6WT ACTN4 knockdown (KD) fibroblasts transiently transfected with indicated ACTN4 plasmids. Live cell tracking of transfected cell grown in complete growth media were performed under Nikon live fluorescent microscopy for 16h. Cell migration speed was analyzed using Metamorph software, n=30. Quantitative results represent at least three independent experiments. (D) Trace and dispersion of cell migration of NR6WT ACTN4 KD fibroblasts transiently expressing WT ACTN4-eGFP and 14–911 ACTN4-eGFP, respectively. Lines are created using Metamorph software, n=20. (E) NR6WT ACTN4 KD fibroblasts transiently expressing WT ACTN4-eGFP or 14–911 ACTN4-eGFP were fixed, permeabilized and then stained with rhodamine phalloidin. Scale bar = 5 µm. Shown are representative images of at least three experiments.