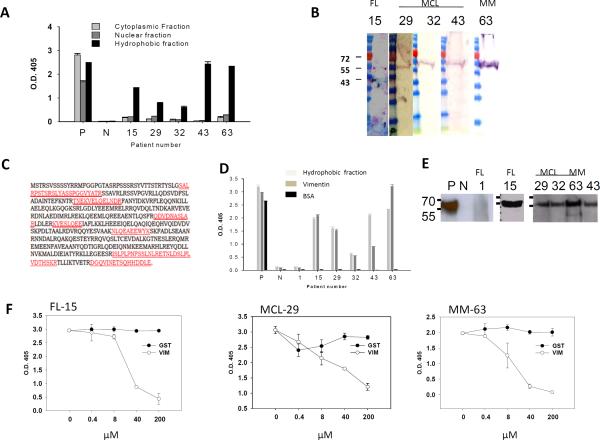
Figure 3. Tumor Igs recognize vimentin.
Tumor Igs from FL (15), MCL (29, 32, 63), and MM (63) were tested in various assays to characterize the antigens they recognized. Polyreactive ED38 antibody was used as a positive control (P), and nonreactive mG053 antibody was used as a negative control (N) where appropriate. Error bars represent the standard deviation for triplicate samples. (A) Tumor Igs were tested in triplicate for reactivity against hydrophilic cytoplasmic and nuclear proteins fractions and a hydrophobic fraction from HEp-2 cells using ELISA as described in Materials and Methods. (B) The hydrophobic fraction from HEp-2 cells was dissolved in urea, subjected to SDS-PAGE, and transferred to nitrocellulose membrane. Immunoblotting was performed with tumor Igs (5 μg/ml), and bound tumor Igs were detected by goat alkaline phosphatase-conjugated antihuman Ig. (C) The corresponding bands (arrows in panel B) were excised from Coomassie Blue-stained gels and subjected to sequential mass spectrometry. Seven peptides (marked in red) that matched the vimentin protein sequence were observed. (D) Tumor Igs were tested in triplicate for reactivity against the HEp-2 hydrophobic fraction, commercially obtained recombinant human vimentin, and BSA (50 μl of 10 μg/ml) using ELISA. (E) Tumor Igs were tested for reactivity against commercially obtained recombinant human vimentin using Western blotting. (F) Tumor Igs were preincubated with various concentrations of recombinant human vimentin or glutathione S-transferase and then tested for reactivity against the HEp-2 hydrophobic fraction.