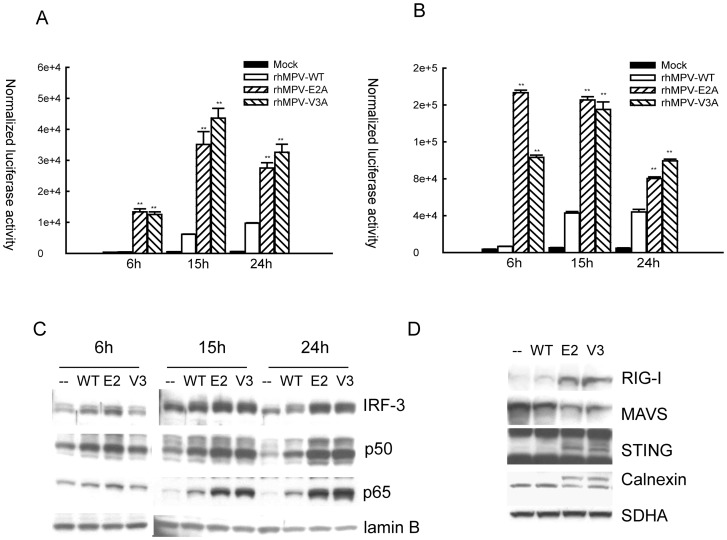
Figure 6. Glu 2 and Val 3 residues of G protein suppress IRF-3 and NF-κB activation by inhibiting mitochondrial signalosome formation.
A549 cells were cotransfected with a luciferase reporter plasmid containing the RANTES ISRE site (A) or multimers of the IL-8 NF-κB site (B). At 24 h post-transfection, cells were infected with rhMPV-WT or rhMPV-E2Aor rhMPV-V3A, at MOI of 2, and harvested at different times p.i. to measure luciferase activity. Uninfected plates served as controls. For each plate luciferase was normalized to the β-galactosidase reporter activity. Data are representative of two independent experiments and are expressed as mean± SE. **, P<0.01, relative to rhMPV-WT-infected A549 cells. (C) A549 cells were infected with rhMPV-WT or rhMPV-E2A, or rhMPV-V3A, at MOI of 2, for various lengths of time and harvested to prepare nuclear extracts. Equal amounts of protein from uninfected and infected cells were analyzed by Western blot using an anti-IRF-3, anti-p50 or anti-p65 antibody. Membranes were stripped and reprobed for lamin b, as control for equal loading of the samples. Data shown are representative of two independent experiments. (D) U4A cells were infected with rhMPV, WT or G mutants and harvested at 6 h p.i. to purify mitochondria. The abundance of mitochondria-associated RIG-I, MAVS, STING and calnexin was investigated by Western blot. Membranes were stripped and reprobed with anti-SDHA, as control for comparable loading of samples. Data shown are representative of two independent experiments.