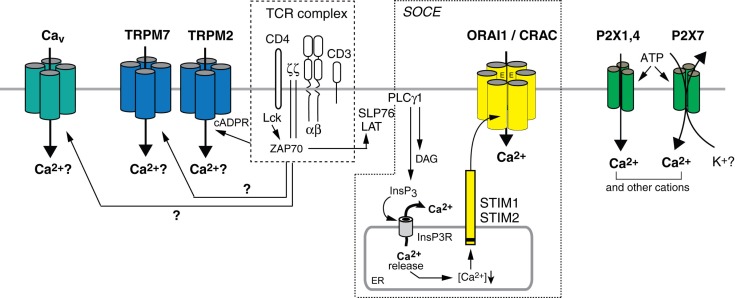
Figure 1.
Ca2+ influx pathways in T cells. Stimulation of T cells through the TCR complex results in Ca2+ influx, which is involved in the regulation of many T cell functions. CRAC channels mediate store-operated Ca2+ entry (SOCE) following activation of PLCγ1 and production of InsP3. InsP3 binds to and opens Ca2+ permeable InsP3 receptors (InsP3R) in the ER, resulting in the release of Ca2+ from ER stores (Lewis, 2001; Feske, 2007). Ca2+ release from the ER causes the activation of STIM 1 and 2, which oligomerize and translocate to ER-plasma membrane junctions. STIM1 and STIM2 bind to ORAI1, the pore-forming subunit of the CRAC channel, thereby mediating its opening and sustained Ca2+ influx. The subunit composition of the CRAC channel awaits further studies; both tetrameric and hexameric assemblies of ORAI1 subunits were proposed (Ji et al., 2008; Mignen et al., 2008; Penna et al., 2008; Maruyama et al., 2009; Hou et al., 2012). TRPM2 is a Ca2+ permeable cation channel that can be activated by cADPR and NAADP in human T cells (Beck et al., 2006). Increased cADPR levels after TCR stimulation (Guse et al., 1999) activate SOCE by releasing Ca2+ from the ER through RyR channels and potentially activate TRPM2 channels directly. TRPM7 is a non-selective cation channel implicated in Mg2+ homeostasis in T cells; whether its ability to conduct Ca2+ contributes to T cell function and how it is activated by TCR stimulation is not understood. The L-type Cav channels Cav1.2, Cav1.3, and Cav1.4, which mediate depolarization-dependent Ca2+ influx in excitable cells including neurons may contribute to Ca2+ influx in T cells but their activation mechanism is unknown and their current properties are not well defined. P2X receptors are non-selective Ca2+ channels activated by extracellular ATP. Several homologs, P2X1, P2X4, and P2X7, were reported to mediate Ca2+ influx in T cells in vitro.