Table 1.
Optimization of the Cyclocarbonylation Reaction Conditions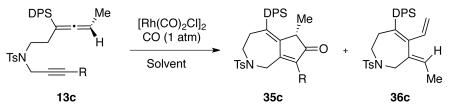
| Entry | [Rh(CO)2CI]2 (mol %) | Time (h) | T(°C) | Product and yield (%) | Solvent |
|---|---|---|---|---|---|
| 1 | 10 | 3 | 90 | 35c (95); 36c (0) | PhMe |
| 2 | 5 | 9 | 90 | 35c (75); 36c (9) | PhMe |
| 3 | 10 | 7 | 120 | 35c (61); 36c (35) | PhMe |
| 4 | 5 | 7 | 120 | 35c (42); 36c (48) | PhMe |
| 5 | 1 | 24 | 120 | 35c (29); 36c (26) | PhMe |
| 6a | 10 | 24 | 40 | 35cb (72); 36c (0) | DCE |
PPh3 (30 mol %), AgBF4 (22 mol %);
Yield based on recovered starting material (conversion : 54%)
