Abstract
We reviewed the extant literature with the goal of assessing the extent to which resting-state functional connectivity is associated with phenotypic variability in healthy and disordered populations. A large corpus of work has accumulated to date (125 studies), supporting the association between intrinsic functional connectivity and individual differences in a wide range of domains—not only in cognitive, perceptual, motoric, and linguistic performance, but also in behavioral traits (e.g., impulsiveness, risky decision making, personality, and empathy) and states (e.g., anxiety and psychiatric symptoms) that are distinguished by cognitive and affective functioning, and in neurological conditions with cognitive and motor sequelae. Further, intrinsic functional connectivity is sensitive to remote (e.g., early-life stress) and enduring (e.g., duration of symptoms) life experience, and it exhibits plasticity in response to recent experience (e.g., learning and adaptation) and pharmacological treatment. The most pervasive associations were observed with the default network; associations were also widespread between the cingulo-opercular network and both cognitive and affective behaviors, while the frontoparietal network was associated primarily with cognitive functions. Associations of somatomotor, frontotemporal, auditory, and amygdala networks were relatively restricted to the behaviors linked to their respective putative functions. Surprisingly, visual network associations went beyond visual function to include a variety of behavioral traits distinguished by affective function. Together, the reviewed evidence sets the stage for testing causal hypothesis about the functional role of intrinsic connectivity and augments its potential as a biomarker for healthy and disordered brain function.
Key words: cingulo-opercular, connectivity networks, default, fMRI, frontoparietal, motor, performance, sensory
Introduction
The recent growth in studies of the resting-state signals a paradigm shift in cognitive neuroscience from examining stimulus-/task-evoked or extrinsic neural activity to examining task-free or intrinsic neural activity (Raichle, 2009). Soon after the discovery of the stimulus-/task-evoked blood oxygen level-dependent (BOLD) response, Biswal and coworkers (1995) observed that slow fluctuations (<0.1 Hz) in the BOLD timecourse were correlated across motor regions in the absence of a stimulus or task. This study was pioneering because it revealed that the coherent rise and fall of spontaneous activity during a task-free state were not random noise, but rather carried a functional representation. Since then, starting with a study of the default-mode network (Greicius et al., 2003), multiple functional networks, commonly termed intrinsic connectivity networks (ICNs), have been identified with high reliability (Shehzad et al., 2009), while subjects were asked to rest and stay awake with their eyes open or closed. These ICNs may reflect Hebbian plasticity induced by the lifetime history of coactivated regions (Dosenbach et al., 2007). While its functional significance is debated, there is high enthusiasm for intrinsic connectivity as a tool for the study of brain function [for reviews, see Greicius (2008); Zhang and Raichle (2010)].
Several key findings provide a strong theoretical foundation for the study of the intrinsic functional architecture of the brain. First, ICNs are unlikely to be a product of conscious cognitive processes, as they are preserved during sleep (Fukunaga et al., 2006) and anesthesia (Greicius et al., 2008; Vincent et al., 2007). Second, their strength and organization change during development (Fair et al., 2008), suggesting that ICNs are shaped by genetic and experience-dependent maturational processes. Third, ICNs parallel anatomic connectivity, but direct anatomic connections are not necessary for intrinsic connectivity (Honey et al., 2009); therefore, intrinsic connectivity likely results from both mono- and multisynaptic relationships. Fourth, the spatial composition of ICNs strongly parallels patterns of activation during tasks evoking specific functions (e.g., visual, motor, language, and attention) (Smith et al., 2009), suggesting that ICNs recapitulate the topography of stimulus-/task-evoked responses. Fifth, intrinsic neural activity predicts individual variability in extrinsic neural activity and associated performance (Fox et al., 2006; Gordon et al., 2012; Mennes et al., 2010), suggesting that intrinsic functional architecture is an analog of the functional repertoire of extrinsic responses (Raichle, 2010; Smith et al., 2009).
In the present article, we examine the extant literature to answer a question of practical and theoretical importance: which phenotypic differences are associated with variability in the intrinsic functional architecture of the brain? This knowledge is necessary for multiple reasons. First, it helps to directly elucidate the functional significance of ICNs. If phenotypical variability is predicted by the strength or organization of ICNs, then it provides a basis for potential causal hypotheses about functional representation within ICNs. Second, it helps to evaluate the potential for ICNs to serve as endophenotypes. Identification of endophenotypes is a necessary step in the search for causes of debilitating psychiatric and neurological disorders. Third, it helps in evaluating the viability of ICNs to be targets for intervention.
The goal of the present review was to provide a status report of the extent to which intrinsic connectivity is sensitive to phenotypic differences. Thus, we have reached for breadth, providing a comprehensive catalog of findings in the area to date. Studies are included that were returned by PUBMED searches as of July 31, 2012, using combinations of the following search words: functional magnetic resonance imaging (fMRI), resting, intrinsic, connectivity, behavior, behavioral, symptoms, individual differences, predicts, predicted, and correlated with. We only included studies that (i) examined associations with behavioral measures across individuals, either healthy or with a psychiatric or neurological diagnosis; (ii) correlated these measures with connectivity measures from an exclusive resting-state run; and (iii) demonstrated results that were statistically significant, passing correction for multiple correction when applied. We have parsed the results by ICNs as a means of data reduction to draw some general conclusions. Further, detailed methods of each study are not described to reduce the scope of the review; rather, general methods are described below and are identified for each study in the Supplementary Tables S1–S11 (Supplementary Data are available online at www.liebertpub.com/brain), and methodological issues pertinent to interpretation of results are discussed in the Discussion section.
The review flows as follows: we first briefly review functional connectivity methods used in the discovery of reported individual differences, and then describe the most common ICNs, followed by a review of the literature organized by broad behavioral domains. For studies examining association with symptoms of psychiatric or neurological disorders, we classify them under the behavioral domain that is most commonly associated with the symptoms [e.g., schizophrenia under executive function, Alzheimer's disease (AD) under memory, and social anxiety disorder under emotional function]. Across studies, some areas are more extensively studied than others; in such cases, we have summarized the results by drawing conclusions, rather than describing results of each study. In cases where studies are few, it is not possible to draw conclusions, and therefore we have presented the results in more detail. In the discussion, we draw some general conclusions about each ICN and discuss emerging themes and some future directions. Throughout, the term connectivity refers to resting-state functional connectivity. Supplementary Tables S1–S11 present a listing of findings organized by ICNs for easy reference.
Functional connectivity methods
Seed-based/pairwise region-of-interest (ROI) connectivity is the most common method of investigating connectivity relationships. The BOLD signal timecourse is averaged from all voxels within one predefined region (the seed), and then correlations are conducted between that timecourse and the timecourses of activity from other areas of the brain, either in a voxel-wise fashion, or pairwise between a priori seed ROIs. The magnitudes of the resulting Pearson's r values (or Z values, after applying the Fisher transformation) are interpreted as the degree of connectivity, with positive r values indicating strong connectivity relationships, r values near zero indicating no relationship, and negative r values indicating competitive relationships [see Van Dijk et al. (2010), for a comprehensive review of this method].
Independent-component analysis (ICA) is a data-driven method of assessing functional connectivity without the need to define a priori seeds. The ICA algorithm attempts to optimally partition the fMRI signal into a set of spatiotemporal components, each of which has a coherently fluctuating timecourse (i.e., the signal timecourse of all voxels included in the component are similar). The result of the ICA is a set of component maps, in which the values within each voxel of a map represent the likelihood that the voxel is a part of that component [see Beckmann and Smith (2004) for detailed review]. The spatial patterns of these components generally agree with connectivity patterns observed using seed-based approaches, and the components have thus been interpreted as representing discrete networks in the brain.
Regional homogeneity (ReHo) is a voxel-wise measure of functional connectivity in which the similarity is assessed between each voxel's timecourse and those of its immediate neighbors. A higher correlation coefficient is interpreted as demonstrating a more homogeneous activity pattern within a small region of the brain [see Zang et al. (2004) for detailed review], and therefore it is considered a measure of local functional connectivity.
Graph theory analysis is an analysis approach used for complex networks in other domains (such as social or computer networks) that describes brain networks as graphs of nodes (brain regions) linked by edges (seed-to-seed connectivity between the regions). Various metrics of the graph's topology can provide information about the network structure. A network's connectivity density is a measure of the average connectivity strength within the network, while the network's clustering coefficient and modularity are measures of functional segregation (i.e., whether discrete subnetworks are present), which may support local processing. By contrast, path length, global efficiency, and local efficiency are measures of functional integration (i.e., the degree to which information transfer is facilitated across the network). The degree to which a network is simultaneously functionally segregated and integrated is measured by its small worldness, a property that is believed to be optimal for complex information processing. The degree distribution is a measure of the overall degree of connectedness of the network, and it may also be a measure of the network's robustness to damage. Finally, the degree centrality of each node within a network is a measure of the importance of that node to efficient information transfer [for more detailed description of graph theoretic approaches and measures in brain connectivity analysis, see Bullmore and Sporns (2009) and Rubinov and Sporns (2010)].
Intrinsic connectivity networks
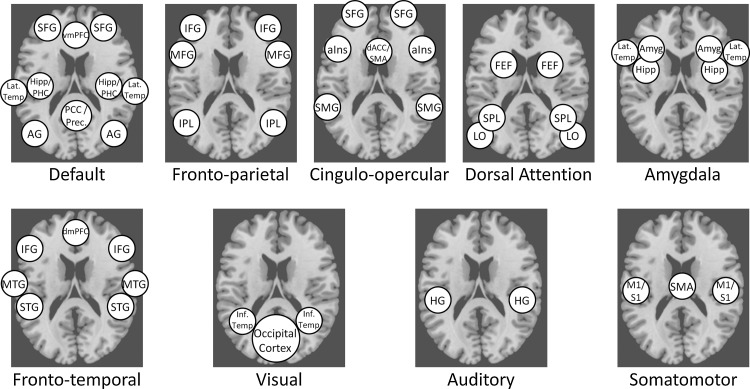
Findings derived from ICA and supported by seed-based methods have converged toward the presence of nine large-scale functional networks in the resting brain, each with a discrete topology (see Figure 1). These networks include a default network; a lateralized frontoparietal network (which has also been referred to as an executive control network); a cingulo-opercular network (also referred to as a salience network, a set-maintenance network, and confusingly, an executive control network); a dorsal attention network; a frontotemporal network (also called a language network); a visual network; an auditory network; a somatomotor network; and an amygdala network. The frontoparietal, cingulo-opercular, and dorsal attention networks have together been referred to as task-positive networks, as they are commonly coactivated during the performance of many externally oriented cognitive tasks. We use these networks as a framework for describing and interpreting brain–behavior associations wherever possible [for more in-depth discussions of these networks and their putative functions, see Beckmann et al. (2005); Damoiseaux et al. (2006); De Luca et al. (2006); Rosazza and Minati (2011); and Smith et al. (2009)]. Additionally, several noncortical areas such as the cerebellum, thalamus, and striatum are not reliably incorporated into any of the above networks. This is likely because nearby subregions and nuclei within these areas are most strongly connected to widely varying networks [cerebellum: Habas et al. (2009); striatum: Di Martino et al. (2008); thalamus: Zhang et al. (2008)], which may prevent accurate regional parcellation by methods such as ICA.
FIG. 1.
Schematic illustration of the central nodes in each of the nine ICNs. AG, angular gyrus; FEF, frontal eye fields; HG, Heschel's gyrus; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; MFG, middle frontal gyrus; MTG, middle temporal gyrus; PCC, posterior cingulate cortex; PHC, parahippocampal cortex; SFG, superior frontal gyrus; SMA, supplementary motor area; SPL, superior parietal lobule; STG, superior temporal gyrus.
Associations by Behavioral Domain
In this section, we summarize results of studies examining associations between resting-state functional connectivity and a variety of behavioral domains. For each behavioral domain, we describe findings in healthy individuals and disordered populations showing associations with measures (e.g., task performance and behavioral ratings) of that domain. An additional section is included for the behavioral domains of executive function, memory, and emotional functions, which reviews findings showing associations with symptoms from disorders that are defined by dysfunction primarily of that behavioral domain.
General intelligence
The concept of general intelligence is useful for describing the integrated functioning of higher cognitive processes such as grasping complex ideas, adapting to the environment, learning from experience, reasoning, and problem solving (Neisser et al., 1996). It is indexed by the Intelligence Quotient (IQ) on the Wechsler Adult Intelligence Scales, or by scores on the Raven's Progressive Matrices and Cattell Culture Fair Test that provide specific indices of the fluid component (Gf) of general intelligence that is independent of acquired knowledge.
Consistent with task-based imaging studies (Jung and Haier, 2007), resting-state studies also show association of intelligence with frontal and parietal regions. Better IQ scores were associated with stronger seed-based connectivity within the frontoparietal network in healthy adults (Song et al., 2008), stronger ReHo within this network (as well as in parahippocampal cortex [PHC], inferior lateral temporal lobe, and fusiform gyrus (FG)] (Wang et al., 2011), and shorter path length within the network (Van den Heuvel et al., 2009). Further, across control and schizophrenic subjects, higher IQ was associated with stronger seed-based connectivity between the frontoparietal network and the cerebellum (Repovs et al., 2011). However, in addition to the frontoparietal network, the default network also appears to be related to general intelligence, as better IQ was predicted by shorter path length within the network (Van den Heuvel et al., 2009) and by reduced connectivity with the frontoparietal network (Song et al., 2008). In addition to within- and cross-network relationships, a study of fluid intelligence points to the importance of the nature of global connectivity of left lateral prefrontal cortex, a part of the frontoparietal network (Cole et al., 2012b). Specifically, higher Gf scores were associated with more positive connectivity within the frontoparietal network and with other task-positive networks, and more negative connectivity with the default, amygdala, auditory, and somatomotor networks.
Summary
Superior intelligence depends upon higher within-network integration and cross-network segregation of the frontoparietal and default networks.
Sensorimotor function
Visual performance
In the context of face processing, greater connectivity between two canonical face-processing regions in the visual network, the occipital face area and the fusiform face area in the FG, predicted a greater face inversion effect, indicating increased holistic processing of faces and superior face recognition abilities in healthy individuals (Zhu et al., 2011). In the context of visual–spatial attention, weaker interhemispheric connectivity of the dorsal attention network (left and right intraparietal sulcus [IPS]) was associated with worse spatial attention performance in patients with right-hemispheric stroke (Carter et al., 2010). Further, inappropriate sensory experience in synesthesia relates to greater connectivity between visual and other networks. In individuals with grapheme-color and phoneme-color synesthesia—a condition in which words and grayscale letters are involuntarily perceived as being colored—abnormally increased connectivity between the visual network and the auditory and frontoparietal networks predicted more consistent synesthetic experiences (Dovern et al., 2012).
Motor performance
Convergent lines of evidence suggest that connectivity within the somatomotor network is associated with motor function. First, greater connectivity within the left than the right side of the somatomotor network (including connections between primary motor cortex [M1], supplementary motor area [SMA], cerebellum, thalamus, and putamen) predicted faster motor performance, less overflow, and less dysrhythmia on a standardized test of motor skills in healthy right-handed children (Barber et al., 2012). Second, weaker interhemispheric connectivity within the somatomotor network predicted worse performance on a variety of hand- and wrist-based motor function measures in patients with stroke (Carter et al., 2010) and less recovery of motor function after 6 months (Park et al., 2011). Third, greater interconnectedness of the somatomotor network (indexed by higher clustering coefficient) predicted faster progression of symptoms of amyotrophic lateral sclerosis (ALS), a progressive disease characterized by degeneration of motor neurons, resulting in rapidly increasing motor weakness and loss of motor control (Verstraete et al., 2010). Thus, while a strongly connected somatomotor network may facilitate motor function, it may also facilitate the spread of ALS symptoms.
In addition to within-network connectivity, connectivity between the somatomotor and other networks is associated with motor function. First, weaker connectivity between the somatomotor network and the contralateral frontoparietal network (and thalamus) predicted less recovery of motor function 6 months after the stroke (Park et al., 2011). Second, a mixed pattern of reduced and increased connectivity degree (a measure of the overall connectedness of a region) of specific nodes of the somatomotor network with other regions predicted the severity of motor symptoms in Parkinson's disease (PD; indexed by the Unified Parkinson's Disease Rating-scale motor score) (Wu et al., 2009). Specifically, worse motor function was associated with reduced connectivity degree of regions that nominally have widespread connectivity with other brain regions (thalamus, putamen, SMA node of the somatomotor network, and frontoparietal network) and increased connectivity degree of regions that usually have focal connectivity (M1, primary somatosensory cortex [S1], premotor nodes of the somatomotor network, and cerebellum). Thus, these findings suggest that degeneration in dopamine signaling in PD may induce motor dysfunction by reducing widespread connectivity and over-generalizing focal connectivity.
Visual–motor integration
Visual–motor processing speed has been associated with the cingulo-opercular, frontoparietal, and default networks. In patients with cognitive dysfunction due to hepatic encephalopathy, a liver disease, reduced connectivity within a frontoparietal ICA component was associated with slower performance on a number trail-making task (Qi et al., 2012). In the same patients, the default ICA component showed associations with both increased and reduced connectivity such that increased posterior connectivity predicted slower performance on the number trail-making task, while reduced anterior connectivity predicted slower digit–symbol matching performance. Further, increased connectivity of the putamen within the cingulo-opercular network was related to slower digit–symbol matching performance across both lean and obese adults (García-García et al., 2012). Higher-order visual–motor integration was associated with the cingulo-opercular network such that increased connectivity between the left anterior insula (aIns) and the rest of the cingulo-opercular network across older and younger adults predicted higher block-design performance indexing visuospatial intelligence (Onoda et al., 2012).
Summary
Individual differences in visual and motor functions were associated with connectivity strength within their respective modality-specific networks, such as visual and somatomotor. Similarly, pathological integration across modalities as in synesthesia was correlated with the extent of connectivity between the relevant modality-specific networks, visual and auditory. Further, shifting visual attention in space was associated with a network encompassing parietal regions known to subserve spatial attention, termed the dorsal attention network. In contrast, integration of visual and motor processes was associated with neither of these modality-specific networks, but rather with networks integrating multiple heteromodal cortical regions such as the cingulo-opercular, frontoparietal, and default networks. Further, connectivity across the somatomotor and frontal-parietal networks was important for predicting loss or recovery of motor function.
Executive function
Executive function is a constellation of processes that support the deployment of cognitive resources in a goal-directed manner; these processes include working memory, attention, inhibitory control, fluency, and task-switching. Individual differences in each of these processes have been repeatedly linked to connectivity within and between the frontoparietal, cingulo-opercular, and default networks, whereas higher scores on a composite measure of these processes (indexed by the Frontal Assessment Battery) were associated with increased connectivity between the dorsal anterior cingulate cortex (dACC) and the rest of the cingulo-opercular network in healthy young and older adults (Onoda et al., 2012).
Working memory
Working memory refers to the ability to maintain and manipulate information for a short period of time. Working memory function was associated with both whole-brain connectivity and with selective networks, including default, frontoparietal, and cingulo-opercular. Global measures such as increased small worldness (network efficiency) predicted superior working memory capacity, and degree of modularity predicted variability in memory capacity across sessions (Stevens et al., 2012a). Superior working memory was also related to increased connectivity within the default network, from ventromedial prefrontal cortex and adjacent regions (vmPFC) to posterior cingulate cortex (PCC) (Hampson et al., 2006, 2010), with increased connectivity of PCC in default-network ICA components (Sala-Llonch et al., 2012), with increased connectivity between the frontoparietal network and the cerebellum across both controls and schizophrenics (Repovs et al., 2011), with increased connectivity between the cingulo-opercular network and the putamen across both controls and schizophrenics (Tu et al., 2012), and with reduced connectivity between default and frontoparietal networks (Hampson et al., 2010). In addition to cortical connectivity, reduced connectivity between subcortical structures such as the thalamus and striatal regions (bilateral globus pallidus and putamen) was associated with better working memory across both children with and without attention-deficit hyperactivity disorder (ADHD) (Mills et al., 2012). Thus, working memory function was associated with stronger connectivity within default and cingulo-opercular regions and also between the frontoparietal network and cerebellum, a region often activated during working memory performance, as well as with weaker connectivity between default and frontoparietal networks and among subcortical regions such as the basal ganglia and thalamus.
Sustained attention
Sustained attention is defined as the ability to consistently maintain attention to goal-relevant stimuli. Individual differences in attention have been linked to both increased and decreased connectivity between the default and task-positive networks. Decreased intertrial variability in response time (an indicator of reduced attention lapses) was linked to less negative connectivity between default and task-positive networks (Kelly et al., 2008). However, better performance on a sustained visual attention task was linked to increased connectivity between the PCC-default node and the inferior frontal gyrus (IFG)/temporal–parietal junction frontoparietal nodes (Pagnoni, 2012). These disparate results suggest that direction of association may differ by the type of attentional process.
Inhibitory control
Connectivity of default, frontoparietal, and cingulo-opercular networks has been associated with variability in inhibitory control. Using the stop-signal task to operationalize response inhibition, better inhibitory control across both healthy children and those with ADHD, a disorder defined by inhibitory deficits, was associated with more positive connectivity among cingulo-opercular nodes (pre-SMA and ACC) and more negative connectivity of cingulo-opercular nodes (ACC and pre-SMA) with nodes of other networks, including subcortical (thalamus) and frontoparietal (middle frontal gyrus [MFG]) nodes (Mennes et al., 2011). Furthermore, diagnosis of ADHD modulated relationships of connectivity and inhibitory performance, such that worse inhibitory control in ADHD children, was related to increased connectivity between cingulo-opercular nodes (pre-SMA, SMA, supramarginal gyrus [SMG], insula, and ACC) and nodes of other networks, including dorsal attention (parietal operculum), visual (lateral occipital cortex), frontoparietal (superior frontal), and striatal (putamen and caudate) nodes. Thus, the cingulo-opercular network and its connectivity with other networks predicted inhibitory control.
In addition to these observed relationships with long-distance connectivity, local connectivity was also associated with stop-signal performance (Tian et al., 2012). Superior inhibitory control was associated with increased ReHo in sensory networks, including visual (bilateral occipital cortex and inferior temporal regions), and auditory (posterior insula [pIns]) and reduced ReHo in default (vmPFC and precuneus) and frontoparietal (bilateral IFG and left inferior parietal lobule [IPL]) network regions.
Inhibitory control in everyday life, indexed by self-reported behaviors reflecting impulsivity, is predicted by cross-network relationships. In healthy adults, increased impulsivity (i.e., worse inhibitory control) as measured by the Barratt Impulsivity Scale was associated with increased density of functional connectivity within visual and default networks, and between cingulo-opercular and amygdala networks (Davis et al., 2012). However, increased impulsivity was also associated with decreased connectivity density between visual and default networks, and between visual and amygdala networks. In patients with Borderline personality disorder, higher impulsivity was associated with increased connectivity of the mPFC node in the default-network ICA component (Wolf et al., 2011). In incarcerated juvenile offenders, bilateral premotor connectivity predicted impulsivity, as measured by the Hare Psychopathy Checklist, such that higher impulsivity was associated with increased connectivity with the default network and reduced connectivity with the dorsal attention and frontoparietal networks (Shannon et al., 2011). In heroin-dependent subjects, higher impulsivity was associated with increased connectivity between the amygdala and the default network, auditory network, and the thalamus, as well as with reduced connectivity between the amygdala and the FG in the visual network (Xie et al., 2011). Thus, across diverse populations, connectivity within the default network, as well as connectivity across networks, was associated with trait-level impulsivity.
Verbal fluency
Fluency refers to the ability to list many objects within a defined category without repeating objects. Across controls and schizophrenics, superior verbal fluency was associated with several metrics of whole-brain connectivity, including average connectivity strength across all regions, clustering, degree distribution, and small worldness (Lynall et al., 2010).
Task switching
A key component of executive control is the ability to flexibly shift from the demands of one task to another. Such task switching has been strongly linked to the frontoparietal network. Superior task-switching abilities have been associated with increased connectivity between bilateral IPL and the rest of the frontoparietal network (Seeley et al., 2007), with increased connectivity between bilateral MFG in children (Gordon et al., 2011), with increased connectivity between frontoparietal network nodes and the cerebellum across controls and schizophrenics (Repovs et al., 2011), and with increased connectivity within frontoparietal ICA components across controls and patients with medial temporal lobe epilepsy (Zhang et al., 2009).
Summary
Two themes emerge from reviewing the large body of work examining associations with executive function. First, in addition to the connectivity of task-positive and default networks, connectivity of subcortical structures such as the striatum, amygdala, thalamus, and cerebellum was also important for predicting individual differences. Second, cross-network relationships were relevant to executive functioning, such that superior function was predicted by more segregation among task-positive networks and between task-positive and default networks in most studies. Further, in some studies, superior function was also associated with more integration between selective task-positive networks (e.g., frontoparietal and dorsal attention), as well as more integration between some networks and subcortical regions (e.g., between visual network and amygdala; between frontoparietal network and cerebellum).
Symptom domains of disorders associated with executive function
Two disorders, ADHD and schizophrenia, have impairment of executive functioning as their primary cognitive sequelae. Their symptomatology cannot be easily parsed into the above executive processes, but rather reflects interactions among behavioral dimensions that comprise executive processes, self-regulation, and emotion.
Attention-deficit hyperactivity disorder
Symptoms of ADHD, such as inattention, hyperactivity, and impulsivity, have been associated with connectivity of default, dorsal attention, and cingulo-opercular networks, as well as among subcortical regions. Higher externalizing symptoms (e.g., aggressive and oppositional defiant behaviors) as measured by the Child Behavior Checklist were associated with reduced positive connectivity within default network regions, including between temporal pole and precuneus, between PCC and superior frontal gyrus (SFG), and between posterior IPL and frontal pole (Chabernaud et al., 2012). Further, connectivity of the left putamen, a subcortical motor region, predicted symptoms measured by the ADHD Rating Scale, such that increased symptoms were associated with reduced connectivity with right subcallosal gyrus/nucleus accumbens and increased connectivity with right global pallidus/thalamus (Cao et al., 2009).
Schizophrenia
Schizophrenia is characterized by the presence of positive symptoms (disrupted or disorganized thought and behavior) and negative symptoms (poverty of speech, thought, or affect), and resting-state connectivity studies have primarily linked individual differences in these symptoms to abnormal connectivity of default and frontoparietal networks.
The severity of positive symptoms has been most frequently linked to increased connectivity between default and frontoparietal networks. More severe positive symptoms were particularly predicted by increased connectivity between default-network nodes (such as vmPFC, PCC, precuneus, and retrosplenial cortex) and lateral frontal frontoparietal nodes (Bluhm et al., 2007, 2009; Skudlarski et al., 2010). Interestingly, while increased connectivity between default and frontoparietal connectivity predicted more severe positive symptoms, it also predicted less-severe negative symptoms (Bluhm et al., 2007; Cole et al., 2011; Venkataraman et al., 2012). This suggests that the positive or negative symptomatic manifestation of schizophrenia may be dependent on the intrinsic connections between these two networks.
More severe positive symptoms were also predicted by increased connectivity between default network and a variety of other networks, including somatomotor, frontotemporal (Bluhm et al., 2007, 2009; Venkataraman et al., 2012), and visual (Bluhm et al., 2009; Meda et al., 2012). Positive symptoms have additionally been linked to connectivity within the default network, though the direction of the effect has not been consistent, with reports of increased symptoms being linked to both reduced (Rotarska-Jagiela et al., 2010; Jang et al., 2011 in healthy relatives of schizophrenics) and increased (Whitfield-Gabrieli et al., 2009) within-network connectivity.
Frontoparietal network connectivity with other networks predicted both positive and negative symptoms. Increased positive symptoms were associated with reduced connectivity within the frontoparietal network (Cole et al., 2011), reduced connectivity between the frontoparietal and auditory networks (Cole et al., 2011) and the cerebellum (Repovs et al., 2011), increased connectivity between frontoparietal and visual/somatomotor networks (Cole et al., 2011), and increased asymmetry of connectivity in the frontoparietal networks (Ke et al., 2010). Increased negative symptoms were associated with increased connectivity between frontoparietal and somatomotor network/cerebellum (Cole et al., 2011), as well as with decreased asymmetry of connectivity in default network (Ke et al., 2010).
Finally, a few studies have suggested that connections not involving the default or frontoparietal networks may be relevant for symptom severity. Increased positive symptoms were predicted by reduced connectivity within the frontotemporal network and by increased connectivity in the auditory network (Rotarska-Jagiela et al., 2010), by reduced connectivity between frontotemporal and somatomotor networks (Skudlarski et al., 2010), and by increased asymmetry of connectivity in cingulo-opercular and visual networks (Ke et al., 2010). Increased negative symptoms were predicted by reduced connectivity between cingulo-opercular network and putamen (Tu et al., 2012), as well as by increased connectivity between amygdala and frontotemporal networks (Meda et al., 2012). More severe negative symptoms were also predicted by greater clustering coefficients and efficiency of the visual network, by longer characteristic path length in the somatomotor network, and by longer path length and reduced efficiency of the whole brain (Yu et al., 2011).
Summary
Individual differences in the severity of symptoms were associated with connectivity of many task-positive and default networks. For both ADHD and schizophrenia, both within-network and cross-network relationships predicted symptom severity. In addition to cortical connectivity, several subcortical regions, including striatal structures and amygdala, correlated with symptoms. In both disorders, these relationships included brain regions that are consistent with affected behavioral domains, including motor (e.g., putamen) and reward (e.g., nucleus accumbens) functions in ADHD, as well as sensory-motor (e.g., somatomotor, visual, and auditory) and emotion (e.g., amygdala) functions in schizophrenia. While studies of ADHD symptom-connectivity were few, those of schizophrenia were many and are riddled with mixed findings, perhaps reflective of the high heterogeneity among patients in the duration of the disorder, medication history, and the nature of behavioral dysfunction.
Learning
Studies examining individual differences in modality/domain-specific learning reveal that such learning is related to short-term plasticity of ICNs, which reflects both the consolidation of recent experience and the predictive value of ICNs measured before learning in determining who will learn better.
Motor learning
Force-field motor learning was associated with connectivity of default, somatomotor, and visual networks and cerebellum (Vahdat et al., 2011). Relative to a prelearning resting scan, increases in the perceptual indices of learning 1 h after force-field learning were associated with increases in connectivity strength within the somatomotor network and between the cerebellum and the mPFC default node, as well as with decreases in connectivity between the cerebellum and visual network. Further, decreases in connectivity between the cerebellum and the somatomotor and visual networks predicted increases in the ability to accurately perform a motor task under force-field conditions.
Visual–spatial learning
Decreases in connectivity strength between the visual network and the frontal eye fields in the dorsal attention network from a prelearning resting scan were associated with greater improvements in accuracy and speed after training on a novel visual discrimination task (searching for T-target among rotated L-distractors for 4 days, 2/3 h/day) (Lewis et al., 2009). These same subjects also showed that the status of prelearning resting-state connectivity of the visual network predicted the amount of subsequent visual learning (Baldassarre et al., 2012). Specifically, the strength of connectivity within the visual network, between heterotopic (i.e., above and below the calcarine sulcus within and across hemispheres), but not homotopic (e.g., above the calcarine across hemispheres) visual regions before training was positively correlated with better learning. Further, the strength of connectivity between visual regions and the left aIns node of the cingulo-opercular network and the vmPFC default-network node was negatively correlated with better learning. Together, these findings suggest that better communication among visual regions and weaker communication between visual and frontal regions involved in detecting and evaluating salient information may facilitate parsing of task-relevant and task-irrelevant visual information in subsequent experience.
Individual differences in navigational ability, as measured by the Santa Barbara Sense of Direction Scale, correlated with learning-related changes in connectivity of the bilateral PHC, a default-network node (Wegman and Janzen, 2011). Specifically, connectivity changes were compared between resting scans performed before and after a route-learning task. Subjects with better navigational ability had increased learning-related connectivity with the right hippocampus, but decreased learning-related connectivity with the right caudate. Thus, consolidation of spatial learning reflected in ICN plasticity depended upon spatial ability.
Pain adaptation
Connectivity of the somatomotor network and the vmPFC default node was associated with individual differences in both retrospective and prospective aspects of learning about painful experience (Riedl et al., 2011). Connectivity in somatosensory and posterior parietal cortex nodes of the somatomotor network correlated positively with pain intensity ratings, both immediately after heat pain was applied to the forearm and after 11 days of exposure to the noxious stimulation. Further, the extent to which subjects adapted to pain over the 11 days correlated positively with connectivity between the vmPFC default-network node and the somatomotor ICA component after the extended exposure to the noxious stimulation, but not before it. Interestingly, this correlation was observed before exposure to the heat pain on the 11th day, which indicates that this somatomotor-default connectivity predicted how much pain the subject anticipated experiencing on the last day.
Summary
Individual variability in learning ability, as well as consolidation of the learning experience, was associated with connectivity of the ICNs associated with the modality or domain of the learning experience (i.e., somatomotor for sensorimotor learning or adaptation and visual for visual–spatial learning). Further, postlearning changes in connectivity also included modality-specific subcortical regions such as the cerebellum for motor function, cortical regions important for set maintenance such as the cingulo-opercular network for visual learning, and the default network.
Memory
Associations between resting-state connectivity and mnemonic function have been observed in healthy subjects as well as in patients with memory disorders.
Autobiographical memory
Subjects who spent a greater percentage of time thinking about their past or future during a resting-scan session had stronger connectivity between the medial temporal default node and other default regions (including vmPFC, restrosplenial cortex, and posterior IPS) (Andrews-Hanna et al., 2010). Thus, at least one function of the default network may be retrieving autobiographical information.
Episodic memory
Studies examining association with episodic memory can be divided into two classes: (i) those examining ICNs after encoding of the to-be-remembered material; and (ii) those examining ICNs before encoding of the to-be-remembered material or some standardized measure of memory ability. These two study types provide different information about memory: the former elucidates circuits that support consolidation of mnemonic information, and the latter elucidates circuits that predict mnemonic ability.
Differences in consolidation are associated with the strength of connectivity between stimulus-selective posterior cortices and frontoparietal and default-network nodes. Immediately after incidental encoding of scenes and faces, better memory for those stimuli was associated with stronger connectivity between right IFG and category-selective seed regions in the default (PHC, for scenes) and visual (FG, for faces) networks (Stevens et al., 2010). Stronger connectivity between hippocampus and right lateral occipital cortex on a resting scan done after encoding of object–face pairs relative to one before encoding predicted better memory for the object–face pairs measured after the scanning sessions (Tambini et al., 2010). A similar brain–behavior relationship was not observed for face–scene pairs, which were not remembered, as well as the object–face pairs. Hippocampal-to-lateral occipital connectivity at baseline did not correlate with subsequent memory performance, which suggests that changes in connectivity during encoding of the material predicted how well the material was later consolidated. Together, these findings suggest that regions involved in the encoding of an experience are sensitive to consolidation of that experience in the time period after the experience.
Differences in mnemonic ability are predicted by higher connectivity within and between default and frontoparietal networks, and lower connectivity among subcortical regions. Better memory performance, as measured by neuropsychological tests such as the Wechsler Memory Scale and California Verbal Learning Test (CVLT), was associated with stronger connectivity between the frontoparietal network and the cerebellum in a combined group of schizophrenic subjects and healthy controls and healthy siblings of both groups (Repovs et al., 2011). Better memory for line drawings of common objects and animals was associated with stronger connectivity between default-network nodes in left and right hippocampi (Wang et al., 2010b). Further, better memory for the face–name pairs and on the Wechsler Memory Scale was associated with stronger connectivity between the hippocampus and PCC/precuneus nodes of the default network in healthy elderly subjects (Wang et al., 2010a). In contrast to these findings of positive correlation with cortical regions, reduced connectivity of subcortical regions (both within thalamus and putamen ICA components and between putamen, thalamus, and caudate components) predicted higher scores on CVLT in elderly subjects (Ystad et al., 2010).
Summary
Connectivity of the default network, particularly between the midline and medial temporal nodes, was important for predicting consolidation of remembered material as well as the ability to remember subsequently encoded material. In addition, these mnemonic functions also depended upon the connectivity of default-network regions with material-specific posterior temporal cortices and the frontoparietal network.
Symptom domains of disorders associated with memory dysfunction
Patients with AD and mild cognitive impairment (MCI), a prodromal stage of AD, as well as those with other disorders whose pathology involves brain structures important for episodic memory, show associations between alterations of ICNs and primary symptoms that are marked by episodic memory dysfunction.
AD and MCI
Reduced connectivity within the default ICA component predicted reduced scores on the Mini-Mental State Examination (MMSE) in patients with AD (Wu et al., 2011b), as did decreased ReHo of the PCC-default node (He et al., 2007). Similarly, in individuals with MCI, reduced PCC-seed- and angular gyrus (AG)-seed-based default connectivity predicted reduced MMSE scores and episodic memory abilities (Liang et al., 2012; Wang et al., 2012a).
The connectivity of several other networks has also been linked to AD symptoms in MCI, possibly reflecting the more variable nature of MCI compared to AD. As in the default network, reduced connectivity within the frontoparietal network predicted reduced MMSE scores (Liang et al., 2011, 2012), while reduced connectivity both within the cingulo-opercular network and between the cingulo-opercular and frontoparietal networks predicted worse episodic memory (Xie et al., 2012a). Additionally, reduced connectivity between default and frontotemporal networks also predicted worse episodic memory, and reduced connectivity between default and frontoparietal networks predicted slower processing speed, but reduced connectivity between default network and caudate predicted better episodic memory (Han et al., 2012).
Interestingly, treatment of patients with AD with the acetylcholinesterase inhibitor donepezil has been shown to reverse these connectivity disruptions, and the degree of connectivity increase has been linked to cognitive improvements. Reductions in AD symptoms were predicted by donepezil-related connectivity increases within the default network (Goveas et al., 2011b; Li et al., 2012a) as well as by connectivity decreases between the hippocampal default node and the frontoparietal and cingulo-opercular networks (Goveas et al., 2011b). This suggests that the observed relationships between connectivity disruptions and symptom severity are highly relevant to disease treatment.
Multiple sclerosis
Multiple sclerosis (MS) is a progressive demyelinating condition that often results in general information-processing deficits as well as episodic memory problems. In patients with MS, worse performance on tests of information processing and episodic memory was associated with reduced connectivity of the PCC and mPFC nodes of a default-network ICA component (Rocca et al., 2010). Further, in patients with MS who underwent cognitive rehabilitation therapy, increases in connectivity between the ACC node of the cingulo-opercular network and the right lateral prefrontal and parietal nodes of the frontoparietal network predicted improvements in information-processing abilities (Parisi et al., 2012).
Traumatic brain injury
Traumatic brain injury (TBI) results from physical trauma to the head, and in mild, closed-head cases, it produces marked, but usually transient, cognitive dysfunction that includes episodic memory problems. In patients with TBI, slower reaction time on a simple choice-reaction time task was predicted by lower connectivity of the PCC within a default-network ICA component (Sharp et al., 2011). Further, worse cognitive symptoms in mild TBI were predicted both by reduced connectivity within the default network and by increased cross-network connectivity between default and frontoparietal networks (Mayer et al., 2011).
However, as TBI-related damage (e.g., diffuse axonal injury) (Scheid et al., 2003) is usually distributed throughout the brain rather than localized to specific regions or networks, ICN alterations ought to be more pervasive. Indeed, patients with mild TBI demonstrated abnormally increased and abnormally asymmetric thalamic connectivity with widespread cortical regions encompassing many networks; the degree of increased connectivity predicted increased memory impairment, while the degree of increased asymmetry predicted increased postconcussive symptoms (Tang et al., 2011). Moreover, in a more comprehensive ICA examining all the brain networks in patients with mild TBI, the degree of postconcussive symptoms was predicted by both increases and decreases in connectivity across virtually all networks, in both within- and across-network connections (Stevens et al., 2012b).
Epilepsy
The chronic nature of epilepsy disrupted the organization of the default network such that patients with higher duration of illness (years from time of first seizure to time of scan) had increased connectivity between the PCC and bilateral parahippocampal regions in the anterior temporal lobes and reduced connectivity of the PCC with dorsomedial prefrontal cortex (dmPFC) (McGill et al., 2012). Reduced rate of retention of verbal information in patients with temporal-lobe epilepsy was associated with increased connectivity between the left medial temporal lobe and PCC in those with seizure focus in the left hemisphere. However, patients with right hemisphere seizure focus appear to compensate, such that higher episodic memory for nonverbal material (faces) was associated with higher connectivity of the intact left medial temporal lobe and anterior mPFC (Doucet et al., 2012).
Wernicke's encephalopathy
Wernicke's encephalopathy, a result of chronic severe alcoholism, includes degeneration of brainstem and medial thalamic nuclei, mammillary bodies, and cerebellum. If left untreated via thiamine replacement therapy, the disease may progress into Korsakoff's syndrome. Within patients undergoing treatment, associations have been found between the severity of verbal memory impairment and the degree of connectivity between the anterior thalamus and mammillary bodies (Kim et al., 2009). The authors suggest that mammilothalamic connectivity may thus index the success of the treatment, suggesting the possibility of individual differences in the reversibility of the disorder.
Summary
In a variety of neurological conditions whose primary symptoms include episodic dysfunction, episodic memory abilities depended upon connectivity within medial temporal, PCC, and mPFC default-network nodes. Further, weaker connectivity between the default network and frontoparietal and cingulo-opercular networks was associated with reduced episodic memory, and response to treatment that improved episodic memory included strengthening of those connections. Further, connectivity of subcortical structures such as thalamus and mammillary bodies was also responsive to treatment, improving memory function.
Language
Greater language competence was associated with higher integration within nodes of the frontotemporal network. Better language function measured by story writing and a standardized test of Mandarin was associated with higher connectivity within the temporal nodes of the frontotemporal network (e.g., ReHo within right superior temporal gyrus [STG], and inter- and intrahemispheric connectivity of the right STG) in congenitally and acquired deaf individuals (Li et al., 2012b). Higher verbal IQ scores were associated with higher connectivity within the IFG in patients with epilepsy with left-sided, but not right-sided, seizure focus (Pravatà et al., 2011). Superior learning of novel syntax on an artificial grammar task was associated with lower interhemispheric connectivity between left and right Broca's area (BA 44/45) and frontotemporal nodes in healthy elderly subjects, suggesting that functional segregation of language-specialized regions is advantageous for language function (Antonenko et al., 2012).
Reading competence was associated with higher connectivity within the frontotemporal network, and with developmental differences in its relationship with other task-positive and default networks. Higher reading scores were associated with increased connectivity among regions important for language (between Broca's and Wernicke's areas within the frontotemporal network and between the precentral gyrus and other regions within the somatomotor network) in both children and adults (Koyama et al., 2011). Further, in adults, superior reading competence was also predicted by increased connectivity between the left FG (visual word-form area [VWFA]) in the visual network, by increased connectivity between left-sided nodes of the frontotemporal and frontoparietal networks, and by decreased connectivity between the VWFA and the vmPFC and PCC default-network nodes. However, in children, these relationships were reversed, such that better reading competence was predicted by decreased VWFA-to-frontotemporal/frontoparietal connectivity, but by increased VWFA-to-default connectivity. Thus, it appears that across-network relationships undergo maturational changes as the development of reading skill progresses.
In contrast to reading competence, better semantic performance was associated with increased connectivity within the frontotemporal network (e.g., left IFG and bilateral anterior temporal lobes), as well as with default-network regions such as the left medial temporal lobe, PCC, dmPFC, and vmPFC (Wei et al., 2012).
Summary
Higher language competence on a variety of indices was associated with stronger connectivity within frontotemporal networks, as well as with its connectivities with other networks. While semantic abilities were associated with more integration of the frontotemporal nodes with default network, reading competence was associated with less integration with default-network nodes, frontoparietal nodes, and extrastriate visual regions. Further, associations between language ability and cross-network relationships were reversed in childhood, suggesting that changes in cross-network relationships define the developmental trajectory of reading competence.
Emotional function
Resting-state connectivity has been associated with global dimensions of emotional functioning (e.g., internalizing and externalizing problems on the Child Behavior Checklist), as well as with single dimensions (e.g., anxiety and empathy) and with personality traits.
Internalizing/externalizing problems
Higher internalizing scores (e.g., behavior reflective of anxiety, depression, and social withdrawal) showed a positive association with stronger connectivity among all default nodes, as well as a negative association with connectivity between default network and both frontoparietal network (anterior mPFC to IFG and premotor cortex) and cingulo-opercular network (retrosplenial cortex to insula, dACC, and SMA) (Chabernaud et al., 2012). Further, higher externalizing scores (e.g., aggressive behaviors) were associated with connectivity between default and task-positive networks, positively with the cingulo-opercular network (AG/posterior IPL to the dACC and SMA) and negatively with the dorsal attention network (restrosplenial cortex to posterior parietal/dorsal occipital cortex). The presence of ADHD moderated connectivity of the default network such that higher externalizing scores in children with ADHD were associated with less-positive connectivity within the default network (temporal poles to PCC, precuneus, and SFG, and AG/posterior IPL to frontal poles), while the opposite relationship was observed in control children.
Anxiety
The level of anxiety experienced at the time of the resting scan alters the connectivity strength of default, cingulo-opercular, and amygdala networks. In healthy subjects, state anxiety measured by the State Trait Anxiety Inventory was positively correlated with right amygdala–dmPFC connectivity and negatively correlated with right amygdala–vmPFC connectivity (Kim et al., 2011). Similarly, a negative correlation with left amygdala–ventral mPFC and PCC/precuneus connectivity was also observed in a combined group of healthy controls and patients with social anxiety disorder or panic disorder (Hahn et al., 2011). Connectivity of the aIns, a cingular-opercular node, was also implicated in two studies in which subjects rated how anxious they felt before or during the scan. First, connectivity from aIns to other cingulo-opercular regions such as dACC and left SFG was stronger in individuals with higher anxiety (Seeley et al., 2007). Second, connectivity between a default ICA component and the left aIns was stronger in adolescents and adults with higher anxiety; additional regions showing a positive correlation included the caudate in adolescents, and the right IFG, left PHC, and left PCC in adults (Dennis et al., 2011).
Empathy
Individual differences in empathy are predicted by differences in connectivity of regions involved in emotional, social-cognitive, and interoceptive functions that comprise the default, cingulo-opercular, frontotemporal, and amygdala networks, as well as brainstem regions. One behavior indicative of empathy is the extent to which one feels another's pain. Ratings of how much one felt the physical pain depicted in pictures correlated positively with connectivity of vmPFC and subgenual ACC regions of the default network (Otti et al., 2010). Empathy can also be classified into affective (e.g., visceral reaction to others' emotional state) and cognitive (e.g., ability to take others' perspective) aspects. Their relative dominance, measured by the Interpersonal Reactivity Index, was predicted by the strength of connectivity among default, cingulo-opercular, and amygdala networks (Cox et al., 2011). Specifically, higher dominance of affective over cognitive aspects was associated with increased connectivity among social–emotional processing regions such as ventral aIns (cingulo-opercular), vmPFC (default), and amygdala, whereas higher relative dominance of cognitive aspects was associated with increased connectivity among social-cognitive and interoceptive regions such as brainstem, superior temporal gyrus (STG) (frontotemporal), and aIns (cingulo-opercular).
Personality traits
Personality research has identified five independent domains representing traits that vary substantially across individuals. These domains, as measured by the NEO-PI-R (Costa and McCrae, 1992), are neuroticism, extraversion, openness to experience, agreeableness, and conscientiousness. These personality domains predicted seed-based connectivity of dACC and precuneus with all major networks, as follows (Adelstein et al., 2011): Neuroticism predicted connectivity with dmPFC, middle temporal gyrus, and temporal pole, regions known to be engaged during self-referential processing and fearful anticipation and negative emotion; extraversion predicted connectivity with limbic regions known to be involved in reward and motivation, as well as FG, known to be involved in face processing; openness to experience predicted connectivity with mPFC, PCC/precuneus, and dorsolateral prefrontal cortex (dlPFC), regions involved in processing of the self and the external environment; agreeableness predicted connectivity with posteromedial extrastriate regions and sensorimotor cortex, regions known to be involved in social and emotional attention; and conscientiousness predicted connectivity with medial temporal lobe regions known to be involved in future-oriented processing. In another study that focused upon agreeableness, a temperament trait thought to be associated with reactivity to conflict, connectivity of the PCC with cingulo-opercular (ACC and SMG) and visual (cuneus, left middle occipital, and right lingual gyrus) networks was positively correlated with agreeableness (Ryan et al., 2011). Further, this study found that higher blood pressure responsivity to cognitive demands was associated with connectivity of the ACC, and that this relationship was mediated by agreeableness.
Some behaviors displayed by people with Autism such as difficulty with social interaction/communication, restricted interests, and repetitive behaviors can be considered personality traits that also vary among healthy individuals. Increased autistic-like traits in healthy individuals (measured by the Social Responsiveness Scale [SRS]) were associated with reduced connectivity of a pregenual ACC-default seed with bilateral aIns (part of the cingulo-opercular network) and increased connectivity with left superior parietal lobule (SPL) and lateral occipital cortex (part of the dorsal attention network) and AG (part of the default network) (Di Martino et al., 2009). However, across both groups of subjects with Autism Spectrum Disorders (ASD) and healthy controls, higher autistic traits (measured by the Autism Spectrum Quotient [AQ]) were associated with reduced connectivity within the default network (vmPFC–bilateral AG), even when group membership was factored out (Von dem Hagen et al., 2012). This finding of negative correlation within default regions with autistic traits conflicts with that of Di Martino and colleagues (2009) showing a positive correlation of autistic traits with connectivity within default regions (PCC-AG) and may be attributable to differences in the measure of autistic traits (AQ being self-report and SRS being other report, usually a significant other/family member) or the choice of seed region [an ICA component in Von dem Hagen et al. (2012), but a region of hypoactivation in previous ASD studies in Di Martino et al. (2009)].
The extent to which one is willing to take risks is a personality trait that is associated with psychopathology, such that low- or high-risk seeking predisposes one to anxiety disorders or pathological gambling/addiction, respectively. Higher risk taking (measured by the Cognitive Appraisal of Risky Events Questionnaire [CARE]) was predicted by reduced connectivity between the frontoparietal right IFG node and the cingulo-opercular right aIns node, as well as by more negative connectivity between the left nucleus accumbens, a region involved in reward and motivation, and parieto-occipital regions belonging to three networks (cingulo-opercular: SMG; dorsal attention: lateral occipital cortex and SPL; default: precuneus and AG) (Cox et al., 2010). Thus, the interaction of these different networks at rest is predicted by a personality trait relevant to decision making, cognitive control, and motivational functions.
Summary
Two themes are evident in the results from studies examining associations between resting-state connectivity and behaviors associated with emotional function: First, connectivity of the default network, both within selective nodes and with task-positive or subcortical regions, was associated with behavior regardless of whether it emphasized unitary emotional functions (e.g., anxiety) or those with multiple dimensions that included motivational and cognitive functions. Second, connections showing brain–behavior associations included regions known to subserve socioemotional or sociocognitive functions based upon task-evoked studies.
Symptom domains of disorders associated with emotional dysfunction
Disrupted socioemotional function is a defining symptom of psychiatric disorders such as mood and anxiety disorders, personality disorder, addiction, developmental disorders such as ASD, and neurological conditions such as frontotemporal dementia. Symptom domains of these disorders were predicted by resting-state connectivity of the default network, multiple task-positive networks, and multiple subcortical structures.
Depression
Among individuals with major depressive disorder (MDD), increased connectivity within the default network has been consistently associated with severity of the disorder. More severe depressive episodes were also predicted by increased node centrality of the hippocampus (Zhang et al., 2011), as well as by greater connectivity between the hippocampus and the rest of the default network (Goveas et al., 2011a) in older adults. Increased rumination—a key feature of MDD—was predicted by increased connectivity between PCC- and vmPFC-default nodes (Berman et al., 2011), as well as between the ICA-generated default-network component and the vmPFC and prefrontal cortex (Zhu et al., 2012). Longer depressive episodes were predicted by greater connectivity between PCC and vmPFC (Greicius et al., 2007), by increased node centrality of the hippocampus (Zhang et al., 2011), and by reduced ReHo of the precuneus default-network node (Wu et al., 2011a), suggesting that increased long-distance default connectivity in MDD might be accompanied by decreased local connectivity.
By contrast, in a minority of studies, increased default connectivity predicted reduced depressive symptoms, particularly in older adults. Increased connectivity between the ICA-generated default-network component and the precuneus/AG predicted less over-general autobiographical memory (Zhu et al., 2012). In a study examining cognitive impairment associated with MDD, reduced connectivity between the PCC and the cerebellum predicted lower episodic memory (measured by recall for complex figures) in older adults with remitted MDD who were carriers of the angiotensin-converting enzyme D gene, a risk genotype for dementia (Wang et al., 2012b).
A few studies have also found associations between MDD symptoms and connectivity of extra-default regions—both between default and non-default regions and outside the default network entirely. Higher connectivity degree of the amygdala (i.e., greater connectivity to other brain regions) was associated with longer duration of illness among adolescents (Jin et al., 2011). While this approach could not reveal which specific amygdalar circuits are abnormally enhanced in MDD, other work has shown that increased severity of depressive symptoms was associated with increased connectivity between amygdala and default-network regions (precuneus, SFG, and lateral temporal cortex) across both controls and patients with MCI (Xie et al., 2012b). Increased connectivity both within frontoparietal network (between MFG and IFG) as well as between frontoparietal and default networks (between MFG and SFG) predicted longer duration and increased severity of depressive episodes (Zhou et al., 2010b). By contrast, increased connectivity within the cingulo-opercular network predicted greater subsequent remission of depressive symptoms, as well as reduced apathy and dysexecutive behavior, after treatment with escitalopram (Alexopoulos et al., 2012). Finally, increased node centrality of the caudate predicted longer and more severe depressive episodes (Zhang et al., 2011), whereas reduced degree and betweenness centrality of the left dlPFC in the frontoparietal network predicted greater early-life stress, a risk factor for later MDD (Cisler et al., 2012).
Social anxiety disorder
Differences in long-range and local connectivity of default, visual, frontoparietal, frontotemporal, and dorsal attention networks are associated with severity of social anxiety, as measured by the Liebowitz social anxiety scale. Higher social anxiety was associated with increased connectivity of the frontoparietal ICA component in right inferior/orbital gyrus and reduced connectivity of frontoparietal and visual networks in left SPL and inferior occipital gyrus, respectively (Liao et al., 2010). Higher social anxiety was also associated with increased connectivity between the mPFC node of the default network and multiple bilateral nodes of the visual network (e.g., calcarine, lingual, FG, and middle occipital gyri) and reduced connectivity between the mPFC and the right STG node of the frontotemporal network (Ding et al., 2011). Further, local connectivity of the visual, dorsal attention, and frontoparietal networks also showed correlations with anxiety (Qiu et al., 2011). Higher social anxiety was associated with increased ReHo in left middle occipital gyrus and cuneus (nodes of the visual network) and bilateral lateral parietal cortex (nodes of the dorsal attention network), and with reduced ReHo in left dmPFC (part of the default network), bilateral dlPFC (part of the frontoparietal network), and putamen.
Generalized anxiety disorder
Lower anxiety measured by the Beck's Anxiety Inventory was associated with higher connectivity between the amygdala and right dlPFC, part of the frontoparietal network, suggesting compensatory mechanisms drawing upon executive control processes (Etkin et al., 2009).
Obsessive compulsive disorder
Connectivity of default and cingulo-opercular networks with subcortical regions predicted obsessive compulsive disorder (OCD) symptoms, as measured by the Yale-Brown Obsessive Compulsive Scale (Y-BOCS). More severe OCD was predicted by reduced connectivity between the right aIns node of the cingulo-opercular network and right thalamus (Stern et al., 2012), as well as by reduced connectivity between left dorsal caudate and the rostral ACC node of the default network (Fitzgerald et al., 2011). Reduced connectivity between the PCC default-network node and the right putamen predicted higher general anxiety on the Beck anxiety inventory score in patients with OCD (Jang et al., 2010). In contrast, increased connectivity between a ventral striatal seed and right anterior orbital frontal cortex (part of the default network) was associated with more severe OCD symptoms (Harrison et al., 2009).
Default network connectivity, both within-network and its connection with the visual network, was also sensitive to OCD symptoms. Higher Y-BOCS scores in patients with OCD and in substance-abuse patients, separately and together, were associated with reduced global connectivity of the mPFC default-network node (Meunier et al., 2012). Further, connectivity within the default network, between the mPFC and PCC nodes, predicted specific dimensions of the Y-BOCS, such that connectivity was positively correlated with the cleaning dimension, but negatively correlated with the obsessions/checking dimension. Additionally, connectivity between the PCC node and visual network regions was positively correlated with the symmetry dimension (Jang et al., 2010).
Borderline personality disorder
Patients with a higher score on the Dissociative Tension Scale had higher connectivity of the left aIns, a cingulo-opercular node, and lower connectivity of the cuneus, a visual network node (Wolf et al. 2011).
Post-traumatic stress disorder
Connectivity of the default network predicted current as well as prospective symptom severity (Lanius et al., 2010). Specifically, increased connectivity between the PCC and pregenual ACC nodes was associated with more severe post-traumatic stress disorder (PTSD) symptoms, as measured by the Clinician-Administered PTSD Scale at the time of scanning. Further, increased connectivity between the PCC node and the right amygdala was associated with more severe symptoms 6 weeks later.
Drug addiction
Substance dependence can alter the functional connectivity of the brain, and that altered connectivity has been shown to predict behavioral consequences of the substance dependence in three different domains. First, the strength of functional connectivity has been associated with the severity of the dependence and with the amount of previous substance use. In nicotine-addicted individuals, reduced connectivity between the cingulo-opercular network and bilateral putamen predicted more severe nicotine addiction (Hong et al., 2009). In individuals addicted to the analgesic ketamine, decreased ReHo in the somatomotor network predicted both increased cravings for ketamine and increased total lifetime usage of ketamine (Liao et al., 2012). In heroin-dependent individuals, greater degree of connectivity in the PHC node of the default network, in putamen, and in the cerebellum was associated with longer previous heroin use, while shorter absolute path length in the same regions was also associated with longer previous heroin use (Yuan et al., 2010).
Second, the strength of connectivity has been associated with success in abstaining from the substance. In alcohol-dependent subjects, a longer time remaining sober was associated with greater local efficiency in the cerebellum (Chanraud et al., 2011), while in nicotine-dependent subjects undergoing nicotine replacement, greater nicotine administration-induced reductions in connectivity between default and cingulo-opercular networks predicted greater reductions in withdrawal symptoms such as difficulty concentrating (Cole et al., 2010).
Third, the strength of connectivity has been associated with the cognitive sequelae of the addiction, with abnormally increased connectivity commonly predicting impaired cognition. In cocaine-dependent individuals, greater connectivity between default and frontoparietal networks predicted worse performance on tasks measuring reward-based decision making (e.g., reversal learning and delay discounting) (Camchong et al., 2011).
Autism spectrum disorders
ASD symptoms are associated with default-network connectivity, although the specific nature of findings is mixed across studies. Worse social interaction measured by the Autism Diagnostic Interview (ADI) was consistently associated with reduced default-network connectivity, including between the PCC and SFG nodes (Monk et al., 2009; Weng et al., 2010), and between lateral temporal and PHC-default nodes (Weng et al., 2010). However, for symptoms in the communication domain, findings vary across studies. Worse social communication, measured by the Autism Disorder Observation Schedule or the SRS, was associated with reduced connectivity within a default ICA component (Assaf et al., 2010) and reduced connectivity between default network and the amygdala, frontotemporal, and dorsal attention networks (Gotts et al., 2012). However, verbal communication, as measured by the ADI, was associated with increased connectivity of the PCC-default node with temporal nodes (bilateral lateral temporal lobe and right PHC), and nonverbal communication was associated with increased connectivity between bilateral SFG and lateral temporal lobe-default nodes (Weng et al., 2010). Findings for repetitive behaviors are also mixed, such that more repetitive behaviors were associated with reduced connectivity between PCC and frontal-temporal default nodes (including bilateral SFG, temporal lobe, vmPFC, and dmPFC) (Weng et al., 2010), but with increased connectivity between PCC- and right hippocampal default nodes (Monk et al., 2009). One important difference across studies is the composition of samples, with some studies including only adults or adolescents or children, and others a wide range including adolescents and adults.
Behavioral-variant frontotemporal dementia
Fronto-temporal dementia is a broad class of neurological disorders involving significant neural degeneration in frontal and temporal lobes. Behavioral-variant frontotemporal dementia is a disorder involving disruptions in social–emotional functioning and control of behavior, which has been linked specifically to degeneration in the cingulo-opercular network (Seeley, 2008). Patients with worse symptoms had reduced connectivity of the cingulo-opercular network and increased connectivity within the default network (Zhou et al., 2010a), suggesting that cingulo-opercular disruption may have resulted in reciprocal enhancements in default function.
Summary
Three general observations emerge across a wide variety of conditions that are defined by emotional dysfunction: First, connectivity of the default network, either within its nodes or with task-positive network regions, was associated with disorder severity. While this may be an artifact of methodological approach, as most studies have focused upon default network regions exclusively, it may also highlight the central role that putative default-network functions such as self-referential processing and autobiographical memory may play in disorders of emotion. Second, connectivity with the cingulo-opercular network was the second most commonly observed network predicting symptom domains. This finding is not surprising, as functions thought to be subserved by the network, including detection of salience, interoceptive processing, and set maintenance, are part of the cognitive sequelae of many of the reviewed disorders. Third, across diverse disorders, symptom domains showed associations with connectivity of visual network regions. While this finding was not expected because visual regions are not commonly implicated in emotional functioning, it highlights the fact that connectivity studies may provide a new insight into behavioral function.
Discussion
There is overwhelming evidence supporting the association between intrinsic functional connectivity and phenotypic variability across widely ranging domains of behavior, in both healthy and disordered populations. Intrinsic connectivity is sensitive not only to variability in cognitive, perceptual, motoric, and linguistic performance, but also to variability in traits (e.g., impulsiveness, risky decision making, personality, and empathy) and states (e.g., anxiety and psychiatric symptoms) that are distinguished by altered cognitive and affective functioning. Further, intrinsic connectivity is sensitive to recent (i.e., learning/practice), remote (e.g., early-life stress), and enduring (i.e., duration of symptoms) experiences, as well as to treatment in both psychiatric and neurological disorders. In general, superior function appears to relate to a balance of integration and segregation among cortical ICNs, as well as to relationships with multiple subcortical structures. Thus, the correlational evidence that has accumulated to date provides a strong foundation for testing causal hypotheses about how intrinsic connectivity contributes to behavior.
Disparities in methods across studies may prevent clear general principles from emerging about which specific relationship is favorable for behavioral functioning. First, most studies use ROI-based approaches for measuring connectivity, either as a seed for voxel-wise correlational analysis or for computing pairwise correlations between regions. Across studies, selection of ROIs can be based on anatomical atlases, task-evoked activation loci, ICA-derived components, and/or coordinates from published resting-state studies. In studies with patients, ROIs are often drawn from regions showing group differences or from a group average. Each of these sources may bias results in specific ways. Second, the treatment of the positive/negative direction of connectivity between regions varies across studies. When positive or negative connectivity is specifically examined, interpreting connectivity–behavior relationships is straightforward, as “increasing/decreasing positive or negative” in relation to behavior can be interpreted within the appropriate scale. However, when methods do not distinguish between positive and negative connectivity, interpretation must be in relative terms, as the scale may include only positive or only negative values. Moreover, negative connectivity must be interpretated with caution, as certain data-processing steps such as regressing out the global signal bias connectivity toward negative values [see for discussion of this issue Chang and Glover (2009); Fox et al., (2009); Murphy et al., (2009)] and also induce variability in regional connectivity in ways that distort the observed connectivity (Saad et al., 2012). Third, studies vary in the application of correction for multiple comparisons, and may correct for the number of correlational tests, ROIs, voxels, or none of the above; many studies report both corrected and uncorrected results. When correction is applied, we have reported only those results in the review. Thus, the reliability of results varies across studies due to this factor. Fourth, several studies show that the degree of anxiety that subjects feel during the scan is an important source of variance in the connectivity of cingulo-opercular and amygdala networks, both within the network and in connections with the default network. These findings argue for removing variance due to state anxiety in data processing in a similar fashion to the treatment of other nuisance covariates. Keeping these methodological issues in mind, we draw some general conclusions regarding each network in the following sections.
Default network
Among all the networks, connections with the default network appear to be most pervasively related to behavior, as well as to psychiatric and neurological conditions. These pervasive associations of the default network may simply be a result of a selection bias in investigation, as it is the most common a priori network of interest across studies. Alternately, the default network may be unique in this respect, because it includes some of the most widely connected regions of the brain. Functionally, the PCC and precuneus have the highest resting metabolism of any brain region (Raichle et al., 2001), perhaps in response to their status as primary hub regions (Buckner et al., 2009; Tomasi and Volkow, 2011) based upon their extensive connectivity with the rest of the brain.
Many findings reviewed here suggest that default-network segregation may be a promising candidate endophenotype for executive function. In healthy subjects, stronger within-network connectivity was associated with better working memory, better inhibitory control, better episodic memory ability, and better spatial learning, whereas less negative connectivity between default and other networks was associated with worse working memory, attention, spatial learning, visual learning, and lower IQ. Thus, higher segregation between the default network and other ICNs is associated with better cognition. This may be because less segregation of the default network has been associated with executive problems such as attention lapses (Weissman et al., 2006), mind wandering (Mason et al., 2007), and stimulus-independent thought (Buckner et al., 2008), both in healthy individuals and also in people with ADHD (Castellanos et al., 2008). Convergently, these findings linking properties of default connectivity to both executive task performance and a disorder of executive control establish it as a promising endophenotype for executive function.
In addition to executive function, default-network connectivity was also associated with a variety of behavioral traits and states. In healthy subjects, stronger default-network connectivity predicted externalizing problems, neuroticism, and empathy for pain, whereas its connectivity with other networks (higher/lower) predicted variability in anxiety, personality, social interaction, and empathy. Task-evoked fMRI studies show that these default regions subserve functions associated with internally oriented cognition, particularly thinking about one's self and others (e.g., autobiographical memory, theory of mind, envisioning the future, and social cognition) (Buckner et al., 2008; Laird et al., 2011). Thus, the widespread default-network associations with behavioral traits and states reviewed here may reflect interindividual differences in how we think and feel about ourselves and others.
Some recent studies have started to make inroads into molecular mechanisms by which default-network connectivity may contribute to the affective and mnemonic aspects of internally oriented cognition. Regarding affective function, the glucocorticoid cortisol, an important modulator of stress-related responsivity, was associated with connectivity of the mPFC. Higher resting cortisol levels predicted stronger negative connectivity between mPFC and amygdala (Veer et al., 2012). After social stress, a higher cortisol stress-related response was associated with stronger connectivity between mPFC and the cingulo-opercular network (Thomason et al., 2011). These findings lead to a testable prediction that cortisol function mediates the association between default-network connectivity and affective behavior. Regarding mnemonic function, amyloid-beta deposition, a pathological marker of AD, a dementia with memory disruption, was associated with default-network disruption in healthy older subjects, such that those with higher burden had lower connectivity among default-network regions (Hedden et al., 2009). If amyloid burden mediates the association of the default network with memory impairment in MCI, a precursor state to AD, resting-state default connectivity could be a useful biomarker for predicting risk for AD.
Frontoparietal network
Among all networks, frontoparietal connectivity was most often associated with cognitive function. This network is posited to be involved in goal-directed behavior in the service of adapting to external stimuli or demands, referred to as adaptive control (Dosenbach et al., 2007) or executive control (Seeley et al., 2007). Indeed, connectivity of this network, either within its nodes or with other networks, was associated with performance on tasks drawing upon working memory, inhibitory control, task switching, attention, memory, reward learning, intelligence, as well as with symptoms of disorders involving disruptions of adaptive/executive control such as schizophrenia, AD, TBI, and ADHD. Motor functioning, which is important for goal-directed behavior, also showed associations with this network in neurological conditions such as PD and stroke. Further, behavioral traits that are distinguished by adaptive/executive control abilities such as risk-taking and impulsiveness also showed associations with the network. Unexpectedly, and less-directly relevant to cognitive abilities, correlations were also observed with symptoms of social anxiety, with personality dimensions, and with the presence of remote and enduring events such as early-life stress and the duration of depressive illness, respectively. Perhaps, these associations reflect the involvement of adaptive/executive control in everyday life activities such as social interaction, which differs by personality and is affected in anxiety/depressive illness and early-life stress.
Cingulo-opercular network
This network is posited to be involved in maintaining and implementing task sets and, in concert with the frontal-parietal network, exerts top-down control over behavior (Dosenbach et al., 2007). Further, this network, particularly its aIns node, has been posited to play a critical role in integrating the functioning of disparate ICNs by generating appropriate responses to salient stimuli and switching between internal and externally oriented modes of functioning (Menon and Uddin, 2010). Consistent with these views, connectivity of cingulo-opercular nodes (primarily dACC or aIns) with other networks and subcortical regions (e.g., amygdala and thalamus) was associated with a wide variety of domains such as cognitive functioning, including working memory, inhibitory control, perceptual and motor functions, and episodic memory; affective functioning, including state anxiety, empathy, risk-taking, social interaction, personality dimensions, and internalizing/externalizing problems; symptoms of psychiatric disorders, including OCD, Borderline personality disorder, ASD, and schizophrenia; and symptoms of neurological disorders, including dementia and AD. The aIns is not functionally homogeneous, as social interaction ability was associated with specifically the middle portion between a ventral and dorsal region within the aIns (Di Martino et al., 2009). Such studies that fractionate heteromodal regions of this network such as anterior cingulate and insula based upon connectivity–behavior relationships are needed to fully elucidate the role of this network in behavior.
Dorsal attention network
This network is posited to be involved in the top-down orienting of visual attention. Relative to other task-positive networks, it was associated with only a limited set of behavioral characteristics, perhaps reflecting a selection bias as a network of lower a priori interest. Consistent with its putative role, dorsal attention network connectivity was associated with functions drawing upon visual attention such as visual learning, stimulus detection, response inhibition, and episodic memory for object stimuli. However, it was also associated with a number of traits drawing upon socioaffective functioning such as personality dimensions, risk-taking, impulsivity, social anxiety, and autistic behaviors. Indeed, differences in attentional orienting are altered in those disorders and may distinguish affective functioning pertinent to those traits. Future work is needed to flesh out the specific role that this network may play more broadly in socioaffective functioning.
Amygdala network
This network is posited to be involved in the processing and regulation of emotional information. Consistent with this role, connectivity of the amygdala was associated with a variety of emotional functions, including depression, anxiety, empathy, and PTSD symptoms. The network was also associated with behavioral impulsivity symptoms, likely because the amygdala regulates arousal, which can adversely affect the cognitive control of impulsive behavior (Brown et al., 2006).
Visual network
One observation emerging out of the reviewed studies that may seem surprising is the association of the visual network with a wide array of affective behaviors. Not surprisingly, connectivity with visual network regions predicted functions drawing upon vision such as reading, object perception, and sensory experience. However, connectivity with visual regions was also associated with individual differences in behavioral dispositions (e.g., impulsiveness and personality dimensions), as well as with symptoms of psychiatric disorders such as schizophrenia, social anxiety, OCD, and Borderline personality disorder. One explanation of these associations may be found in studies showing that affective state alters visual attention and perception. Specifically, behavioral studies showed that experimentally induced positive or negative mood expanded or narrowed the field of visual attention, respectively (Rowe et al., 2007), and that expressing fear in facial expression increased subjective visual fields and sped up saccadic eye movements (Susskind et al., 2008). Task-based fMRI showed that these effects of mood on the field of visual attention were mediated by functional connectivity between parahippocampal and visual cortices (Schmitz et al., 2009). Further, viewing emotional images influenced visual event-related potentials differentially such that fearful images augmented perception and attention, whereas disgusting images suppressed it (Krusemark and Li, 2011). Thus, the observed associations with visual network connectivity may reflect enduring effects on visual function induced by the affective states manifested in behavioral dispositions in healthy individuals and in psychiatric disorders.
Other networks
The frontotemporal and somatomotor networks were associated with a focal set of domains consistent with their putative roles in linguistic and sensorimotor function, respectively. The auditory network showed very limited associations with behavioral functions, as it was only related to phoneme–color synesthesia, which may be expected due to the abnormal entanglement of sensory processing with cognition in that condition, and to impulsivity in heroin-dependent subjects, a result that is less easily explained. One notable observation is that all three networks showed association with schizophrenia symptoms. In light of all the other networks' association with schizophrenia, it confirms the characterization of schizophrenia as a disorder with ubiquitous alteration of neural communication (Schmitt et al., 2011). The sensitivity of all cortical ICNs to symptomatic variability in the disorder supports its neurodevelopmental etiology and course, a view that is increasingly supported by molecular genetic studies (Gejman et al., 2011). Another domain with widespread association across all ICNs, except for auditory and frontotemporal networks, was personality dimensions. While these results come from only two studies, with one examining agreeableness (Ryan et al., 2011) and the other examining more dimensions (Adelstein et al., 2011), these results emphasize the sensitivity of intrinsic connectivity to broad phenotypic variability within healthy populations.
Connectivity of multiple subcortical regions such as thalamus, cerebellum, and striatum, including their connectivity with all ICNs, were also pervasively related to a wide variety of behaviors. This is not surprising, considering that cortical control of behavior is implemented via multiple frontal-striatal-thalamic-cerebellar circuits encompassing those regions.
Concluding Remarks
The large volume of resting-state studies accumulated to date shows associations of intrinsic connectivity with a wide variety of cognitive and affective functions, behavioral traits and states, and characteristics of psychiatric and neurological disorders. Most often, ICN involvement was consistent with their known role in behavior based upon task-evoked functional imaging. Thus, the observed associations with phenotypic variability support the notion that the intrinsic functional architecture reflects our extrinsic functional repertoire (although with some limitations) (see Mennes et al., 2012). It also lends credence to the idea that ICNs may reflect the outcome of Hebbian plasticity (Dosenbach et al., 2007), both in the short term (e.g., postlearning) and in the long term (e.g., association with behavioral traits). Further, in many cases, ICN involvement was more widespread than that revealed by task-evoked fMRI studies. For instance, the visual network and dorsal attention networks were associated with affective functions and symptoms of disorders with socioemotional dysfunction. Regions comprising these networks are not usually activated during socioaffective tasks, perhaps due to the choice of control tasks. Together, resting-state connectivity findings confirmed what we know from task-based fMRI, as well as provided novel insights into functional neural organization. Indeed, causal conclusions cannot be drawn from these correlational findings, but the present evidence sets the stage for testing those causal hypotheses.
We end the review with a few concluding thoughts for the future. First, the correlational evidence reviewed here provides the first step needed for formulating pathway models, which trace effects from genes to physiology to neural communication to phenotypes of healthy function and disorders. Indeed, this work is already underway in some areas in which connectivity–behavior relationships have been well established. For example, recent studies combining fMRI and ligand-based imaging provide insight into the dopaminergic mechanisms influencing the associations between default-/task-positive network connectivity and working memory. These studies show that degree of default-/task-positive network segregation depends upon dopaminergic function, as midbrain D3 receptor availability (Cole et al., 2012a), dopamine synthesis capacity (Dang et al., 2012), caudate D1 receptor density (Rieckmann et al., 2011), and genetic polymorphisms of a dopamine-regulating gene (Gordon et al., 2012) were all associated with connectivity of default and task-positive networks. Further, these differences were predictive of executive function, as the dopamine-related effects on connectivity were associated with working memory performance (Rieckmann et al., 2011) and with self-reported impulsivity and distractibility (Gordon et al., 2012).
Second, ICNs' acute sensitivity to variability in both narrow-band (e.g., working or episodic memory task performance) and broad-band (e.g., personality and duration of psychiatric/neurological illness) phenotypic characteristics provides a rich source of candidates for the development of prognostic and protective tools. For instance, if weaker default-network organization is associated with subsequent PTSD, it provides a clue as to which individuals are more susceptible to the disorder. If better function is associated with strong connectivity of specific networks and weaker connectivity of others, behavioral or pharmacological intervention could aim for those targets in populations with disorders of those functions. Further, ICNs appear to be sensitive dependent measures for tracking intervention efficacy and predicting treatment response, based on observed changes after treatment in MDD, AD, and substance abstinence.
Third, thus far, the extant literature has focused on one property of intrinsic connectivity, its topology based on central tendency. When probed for its temporal characteristics, networks show dynamic organization both during task-evoked function and during the resting state. Through the acquisition of a motor skill, networks reorganized dynamically, such that some nodes remained within networks, but others were more flexible, that is, shifted dynamically across networks, and most importantly, flexibility predicted learning (Bassett et al., 2011). A similar dynamic organization has also been observed in the resting state (Chang and Glover, 2010), and importantly, this organization showed differences in characteristics of the mPFC and PCC nodes of the default network between patients with AD and healthy controls (Jones et al., 2012). Thus, other properties such as dynamic organization of ICNs are likely to provide candidates for endophenotypes in the future.
Fourth, findings reviewed here shed new light on the utility of resting-state connectivity for revealing the neural correlates of cognitive function relative to task-evoked fMRI. As resting-state connectivity measures are not confounded by effort or strategy associated with specific cognitive challenges, observed patterns of connectivity likely include regions that reflect an enduring, rather than transient, functional organization of the brain. Therefore, resting-state studies have the potential to reveal networks of regions associated with individual differences in abilities or traits.
In sum, widespread association with phenotypic variability suggests that enthusiasm for resting-state studies is well grounded. The next generation of studies holds great promise for the discovery of mechanistic principles that will put the two modes of neural activity, extrinsic and intrinsic, on par in the study of human behavior.
Supplementary Material
Acknowledgments and Support
This work was supported by the National Institute of Mental Health (MH086709 and MH084961 to C.J.V., and MH088066 to E.M.G.).
Author Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- Adelstein JS. Shehzad Z. Mennes M. DeYoung CG. Zuo X-N. Kelly C. Margulies DS. Bloomfield A. Gray JR. Castellanos FX. Milham MP. Personality is reflected in the brain's intrinsic functional architecture. PLoS One. 2011;6:e27633. doi: 10.1371/journal.pone.0027633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS. Hoptman MJ. Kanellopoulos D. Murphy CF. Lim KO. Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR. Reidler JS. Huang C. Buckner RL. Evidence for the default network's role in spontaneous cognition. J Neurophysiol. 2010;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenko D. Meinzer M. Lindenberg R. Witte AV. Flöel A. Grammar learning in older adults is linked to white matter microstructure and functional connectivity. Neuroimage. 2012;62:1667–1674. doi: 10.1016/j.neuroimage.2012.05.074. [DOI] [PubMed] [Google Scholar]
- Assaf M. Jagannathan K. Calhoun VD. Miller L. Stevens MC. Sahl R. O'Boyle JG. Schultz RT. Pearlson GD. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53:247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarre A. Lewis CM. Committeri G. Snyder AZ. Romani GL. Corbetta M. Individual variability in functional connectivity predicts performance of a perceptual task. Proc Natl Acad Sci U S A. 2012;109:3516–3521. doi: 10.1073/pnas.1113148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AD. Srinivasan P. Joel SE. Caffo BS. Pekar JJ. Mostofsky SH. Motor “dexterity”?: Evidence that left hemisphere lateralization of motor circuit connectivity is associated with better motor performance in children. Cereb Cortex. 2012;22:51–59. doi: 10.1093/cercor/bhr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS. Wymbs NF. Porter MA. Mucha PJ. Carlson JM. Grafton ST. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci U S A. 2011;108:7641–7646. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF. DeLuca M. Devlin JT. Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF. Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Berman MG. Peltier S. Nee DE. Kross E. Deldin PJ. Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6:548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB. Yetkin FZ. Haughton VM. Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bluhm RL. Miller J. Lanius RA. Osuch EA. Boksman K. Neufeld R. Theberge J. Schaefer B. Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL. Miller J. Lanius RA. Osuch EA. Boksman K. Neufeld RWJ. Théberge J. Schaefer B. Williamson PC. Retrosplenial cortex connectivity in schizophrenia. Psychiatry Res Neuroimaging. 2009;174:17–23. doi: 10.1016/j.pscychresns.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Brown SM. Manuck SB. Flory JD. Hariri AR. Neural basis of individual differences in impulsivity: contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6:239–245. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Andrews-Hanna JR. Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Sepulcre J. Talukdar T. Krienen FM. Liu H. Hedden T. Andrews-Hanna JR. Sperling RA. Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E. Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Camchong J. MacDonald AW. Nelson B. Bell C. Mueller BA. Specker S. Lim KO. Frontal hyperconnectivity related to discounting and reversal learning in cocaine subjects. Biol Psychiatry. 2011;69:1117–1123. doi: 10.1016/j.biopsych.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. Cao Q. Long X. Sun L. Sui M. Zhu C. Zuo X. Zang Y. Wang Y. Abnormal resting-state functional connectivity patterns of the putamen in medication-naïve children with attention deficit hyperactivity disorder. Brain Res. 2009;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Carter AR. Astafiev SV. Lang CE. Connor LT. Rengachary J. Strube MJ. Pope DLW. Shulman GL. Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67:365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX. Margulies DS. Kelly AMC. Uddin LQ. Ghaffari M. Kirsch A. Shaw D. Shehzad Z. Di Martino A. Biswal BB. Sonuga-Barke EJS. Rotrosen J. Adler LA. Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabernaud C. Mennes M. Kelly C. Nooner K. Di Martino A. Castellanos FX. Milham MP. Dimensional brain-behavior relationships in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71:434–442. doi: 10.1016/j.biopsych.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2010;50:81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S. Pitel A-L. Pfefferbaum A. Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex. 2011;21:2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM. James GA. Tripathi S. Mletzko T. Heim C. Hu XP. Mayberg HS. Nemeroff CB. Kilts CD. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychol Med. 2012;43:507–518. doi: 10.1017/S0033291712001390. [DOI] [PubMed] [Google Scholar]
- Cole DM. Beckmann CF. Long CJ. Matthews PM. Durcan MJ. Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010;52:590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Cole DM. Beckmann CF. Searle GE. Plisson C. Tziortzi AC. Nichols TE. Gunn RN. Matthews PM. Rabiner EA. Beaver JD. Orbitofrontal connectivity with resting-state networks is associated with midbrain dopamine D3 receptor availability. Cereb Cortex. 2012a;22:2784–2793. doi: 10.1093/cercor/bhr354. [DOI] [PubMed] [Google Scholar]
- Cole MW. Anticevic A. Repovs G. Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 2011;70:43–50. doi: 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW. Yarkoni T. Repovš G. Anticevic A. Braver TS. Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J Neurosci. 2012b;32:8988–8999. doi: 10.1523/JNEUROSCI.0536-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT. McCrae RR. Neo PI-R Professional Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Cox CL. Gotimer K. Roy AK. Castellanos FX. Milham MP. Kelly C. Your resting brain CAREs about your risky behavior. PLoS One. 2010;5:e12296. doi: 10.1371/journal.pone.0012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL. Uddin LQ. Di Martino A. Castellanos FX. Milham MP. Kelly C. The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain's intrinsic functional dynamics. Soc Cogn Affect Neurosci. 2011;7:727–737. doi: 10.1093/scan/nsr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS. Rombouts SA. Barkhof F. Scheltens P. Stam CJ. Smith SM. Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang LC. O'Neil JP. Jagust WJ. Dopamine supports coupling of attention-related networks. J Neurosci. 2012;32:9582–9587. doi: 10.1523/JNEUROSCI.0909-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FC. Knodt AR. Sporns O. Lahey BB. Zald DH. Brigidi BD. Hariri AR. Impulsivity and the modular organization of resting-state neural networks. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs126. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M. Beckmann CF. De Stefano N. Matthews PM. Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Dennis EL. Gotlib IH. Thompson PM. Thomason ME. Anxiety modulates insula recruitment in resting-state functional magnetic resonance imaging in youth and adults. Brain Connect. 2011;1:245–254. doi: 10.1089/brain.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A. Scheres A. Margulies DS. Kelly AMC. Uddin LQ. Shehzad Z. Biswal B. Walters JR. Castellanos FX. Milham MP. Functional connectivity of human striatum: a resting state fMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Di Martino A. Shehzad Z. Kelly C. Roy AK. Gee DG. Uddin LQ. Gotimer K. Klein DF. Castellanos FX. Milham MP. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry. 2009;166:891–899. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J. Chen H. Qiu C. Liao W. Warwick JM. Duan X. Zhang W. Gong Q. Disrupted functional connectivity in social anxiety disorder: a resting-state fMRI study. Magn Res Imaging. 2011;29:701–711. doi: 10.1016/j.mri.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF. Fair DA. Miezin FM. Cohen AL. Wenger KK. Dosenbach RAT. Fox MD. Snyder AZ. Vincent JL. Raichle ME. Schlaggar BL. Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G. Osipowicz K. Sharan A. Sperling MR. Tracy JI. Extratemporal functional connectivity impairments at rest are related to memory performance in mesial temporal epilepsy. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22059. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovern A. Fink GR. Fromme ACB. Wohlschläger AM. Weiss PH. Riedl V. Intrinsic network connectivity reflects consistency of synesthetic experiences. J Neurosci. 2012;32:7614–7621. doi: 10.1523/JNEUROSCI.5401-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A. Prater KE. Schatzberg AF. Menon V. Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA. Cohen AL. Dosenbach NUF. Church JA. Miezin FM. Barch DM. Raichle ME. Petersen SE. Schlaggar BL. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD. Welsh RC. Stern ER. Angstadt M. Hanna GL. Abelson JL. Taylor SF. Developmental alterations of frontal-striatal-thalamic connectivity in obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:938–948.e3. doi: 10.1016/j.jaac.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD. Snyder AZ. Zacks JM. Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Fox MD. Zhang D. Snyder AZ. Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga M. Horovitz SG. Van Gelderen P. De Zwart JA. Jansma JM. Ikonomidou VN. Chu R. Deckers RHR. Leopold DA. Duyn JH. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imaging. 2006;24:979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- García-García I. Jurado MÁ. Garolera M. Segura B. Sala-Llonch R. Marqués-Iturria I. Pueyo R. Sender-Palacios MJ. Vernet-Vernet M. Narberhaus A. Ariza M. Junqué C. Alterations of the salience network in obesity: a resting-state fMRI study. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22104. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gejman PV. Sanders AR. Kendler KS. Genetics of schizophrenia: new findings and challenges. Annu Rev Genomics Hum Genet. 2011;12:121–144. doi: 10.1146/annurev-genom-082410-101459. [DOI] [PubMed] [Google Scholar]
- Gordon EM. Lee PS. Maisog JM. Foss-Feig J. Billington ME. VanMeter J. Vaidya CJ. Strength of default mode resting state connectivity relates to white matter integrity in children. Dev Sci. 2011;14:738–751. doi: 10.1111/j.1467-7687.2010.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM. Stollstorff M. Devaney JM. Bean S. Vaidya CJ. Effect of dopamine transporter genotype on intrinsic functional connectivity depends on cognitive state. Cereb Cortex. 2012;22:2182–2196. doi: 10.1093/cercor/bhr305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM. Stollstorff M. Vaidya CJ. Using spatial multiple regression to identify intrinsic connectivity networks involved in working memory performance. Hum Brain Mapp. 2012;33:1536–1552. doi: 10.1002/hbm.21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ. Simmons WK. Milbury LA. Wallace GL. Cox RW. Martin A. Fractionation of social brain circuits in autism spectrum disorders. Brain. 2012;135(Pt 9):2711–2725. doi: 10.1093/brain/aws160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goveas J. Xie C. Wu Z. Douglas Ward B. Li W. Franczak MB. Jones JL. Antuono PG. Yang Z. Li S-J. Neural correlates of the interactive relationship between memory deficits and depressive symptoms in nondemented elderly: resting fMRI study. Behav Brain Res. 2011a;219:205–212. doi: 10.1016/j.bbr.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goveas JS. Xie C. Ward BD. Wu Z. Li W. Franczak M. Jones JL. Antuono PG. Li S-J. Recovery of hippocampal network connectivity correlates with cognitive improvement in mild Alzheimer's disease patients treated with donepezil assessed by resting-state fMRI. J Magn Reson Imaging. 2011b;34:764–773. doi: 10.1002/jmri.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;24:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD. Flores BH. Menon V. Glover GH. Solvason HB. Kenna H. Reiss AL. Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD. Kiviniemi V. Tervonen O. Vainionpää V. Alahuhta S. Reiss AL. Menon V. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD. Krasnow B. Reiss AL. Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C. Kamdar N. Nguyen D. Prater K. Beckmann CF. Menon V. Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A. Stein P. Windischberger C. Weissenbacher A. Spindelegger C. Moser E. Kasper S. Lanzenberger R. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56:881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Hampson M. Driesen N. Roth JK. Gore JC. Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imaging. 2010;28:1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M. Driesen NR. Skudlarski P. Gore JC. Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SD. Arfanakis K. Fleischman DA. Leurgans SE. Tuminello ER. Edmonds EC. Bennett DA. Functional connectivity variations in mild cognitive impairment: associations with cognitive function. J Int Neuropsychol Soc. 2012;18:39–48. doi: 10.1017/S1355617711001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ. Soriano-Mas C. Pujol J. Ortiz H. López-Solà M. Hernández-Ribas R. Deus J. Alonso P. Yücel M. Pantelis C. Menchon JM. Cardoner N. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- He Y. Wang L. Zang Y. Tian L. Zhang X. Li K. Jiang T. Regional coherence changes in the early stages of Alzheimer's disease: a combined structural and resting-state functional MRI study. Neuroimage. 2007;35:488–500. doi: 10.1016/j.neuroimage.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Hedden T. Van Dijk KRA. Becker JA. Mehta A. Sperling RA. Johnson KA. Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ. Sporns O. Cammoun L. Gigandet X. Thiran JP. Meuli R. Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE. Gu H. Yang Y. Ross TJ. Salmeron BJ. Buchholz B. Thaker GK. Stein EA. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JH. Jung WH. Choi J-S. Choi C-H. Kang D-H. Shin NY. Hong KS. Kwon JS. Reduced prefrontal functional connectivity in the default mode network is related to greater psychopathology in subjects with high genetic loading for schizophrenia. Schizophr Res. 2011;127:58–65. doi: 10.1016/j.schres.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Jang JH. Kim J-H. Jung WH. Choi J-S. Jung MH. Lee J-M. Choi C-H. Kang D-H. Kwon JS. Functional connectivity in fronto-subcortical circuitry during the resting state in obsessive-compulsive disorder. Neurosci Lett. 2010;474:158–162. doi: 10.1016/j.neulet.2010.03.031. [DOI] [PubMed] [Google Scholar]
- Jin C. Gao C. Chen C. Ma S. Netra R. Wang Y. Zhang M. Li D. A preliminary study of the dysregulation of the resting networks in first-episode medication-naive adolescent depression. Neurosci Lett. 2011;503:105–109. doi: 10.1016/j.neulet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Jones DT. Vemuri P. Murphy MC. Gunter JL. Senjem ML. Machulda MM. Przybelski SA. Gregg BE. Kantarci K. Knopman DS. Boeve BF. Petersen RC. Jack CR. Non-Stationarity in the “resting brain's” modular architecture. PLoS One. 2012;7:e39731. doi: 10.1371/journal.pone.0039731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE. Haier RJ. The parieto-frontal integration theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 2007;30:135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Ke M. Zou R. Shen H. Huang X. Zhou Z. Liu Z. Xue Z. Hu D. Bilateral functional asymmetry disparity in positive and negative schizophrenia revealed by resting-state fMRI. Psychiatry Res. 2010;182:30–39. doi: 10.1016/j.pscychresns.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Kelly AMC. Uddin LQ. Biswal BB. Castellanos FX. Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim E. Ku J. Namkoong K. Lee W. Lee KS. Park J-Y. Lee SY. Kim J-J. Kim SI. Jung Y-C. Mammillothalamic functional connectivity and memory function in Wernicke's encephalopathy. Brain. 2009;132(Pt 2):369–376. doi: 10.1093/brain/awn311. [DOI] [PubMed] [Google Scholar]
- Kim MJ. Gee DG. Loucks RA. Davis FC. Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS. Di Martino A. Zuo X-N. Kelly C. Mennes M. Jutagir DR. Castellanos FX. Milham MP. Resting-state functional connectivity indexes reading competence in children and adults. J Neurosci. 2011;31:8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusemark EA. Li W. Do all threats work the same way? divergent effects of fear and disgust on sensory perception and attention. J Neurosci. 2011;31:3429–3434. doi: 10.1523/JNEUROSCI.4394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR. Fox PM. Eickhoff SB. Turner JA. Ray KL. McKay DR. Glahn DC. Beckmann CF. Smith SM. Fox PT. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA. Bluhm RL. Coupland NJ. Hegadoren KM. Rowe B. Théberge J. Neufeld RWJ. Williamson PC. Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Lewis CM. Baldassarre A. Committeri G. Romani GL. Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. Antuono PG. Xie C. Chen G. Jones JL. Ward BD. Franczak MB. Goveas JS. Li S-J. Changes in regional cerebral blood flow and functional connectivity in the cholinergic pathway associated with cognitive performance in subjects with mild Alzheimer's disease after 12-week donepezil treatment. Neuroimage. 2012a;60:1083–1091. doi: 10.1016/j.neuroimage.2011.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Booth JR. Peng D. Zang Y. Li J. Yan C. Ding G. Altered intra- and inter-regional synchronization of superior temporal cortex in deaf people. Cereb Cortex. 2012b doi: 10.1093/cercor/bhs185. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P. Wang Z. Yang Y. Jia X. Li K. Functional disconnection and compensation in mild cognitive impairment: evidence from DLPFC connectivity using resting-state fMRI. PLoS One. 2011;6:e22153. doi: 10.1371/journal.pone.0022153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P. Wang Z. Yang Y. Li K. Three subsystems of the inferior parietal cortex are differently affected in mild cognitive impairment. J Alzheimer's Dis. 2012;30:475–487. doi: 10.3233/JAD-2012-111721. [DOI] [PubMed] [Google Scholar]
- Liao W. Chen H. Feng Y. Mantini D. Gentili C. Pan Z. Ding J. Duan X. Qiu C. Lui S. Gong Q. Zhang W. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage. 2010;52:1549–1558. doi: 10.1016/j.neuroimage.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Liao Y. Tang J. Fornito A. Liu T. Chen X. Chen H. Xiang X. Wang X. Hao W. Alterations in regional homogeneity of resting-state brain activity in ketamine addicts. Neurosci Lett. 2012;522:36–40. doi: 10.1016/j.neulet.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Lynall M-E. Bassett DS. Kerwin R. McKenna PJ. Kitzbichler M. Muller U. Bullmore E. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF. Norton MI. Van Horn JD. Wegner DM. Grafton ST. Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR. Mannell MV. Ling J. Gasparovic C. Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp. 2011;32:1825–1835. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill ML. Devinsky O. Kelly C. Milham M. Castellanos FX. Quinn BT. Dubois J. Young JR. Carlson C. French J. Kuzniecky R. Halgren E. Thesen T. Default mode network abnormalities in idiopathic generalized epilepsy. Epilepsy Behav. 2012;23:353–359. doi: 10.1016/j.yebeh.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA. Gill A. Stevens MC. Lorenzoni RP. Glahn DC. Calhoun VD. Sweeney JA. Tamminga CA. Keshavan MS. Thaker G. Pearlson GD. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry. 2012;71:881–889. doi: 10.1016/j.biopsych.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M. Kelly C. Colcombe S. Castellanos FX. Milham MP. The extrinsic and intrinsic functional architectures of the human brain are not equivalent. Cereb Cortex. 2012;23:223–229. doi: 10.1093/cercor/bhs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M. Kelly C. Zuo X-N. Di Martino A. Biswal BB. Castellanos FX. Milham MP. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50:1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M. Vega Potler N. Kelly C. Di Martino A. Castellanos FX. Milham MP. Resting State functional connectivity correlates of inhibitory control in children with attention-deficit/hyperactivity disorder. Front Psychiatry. 2011;2:83. doi: 10.3389/fpsyt.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier D. Ersche KD. Craig KJ. Fornito A. Merlo-Pich E. Fineberg NA. Shabbir SS. Robbins TW. Bullmore ET. Brain functional connectivity in stimulant drug dependence and obsessive–compulsive disorder. Neuroimage. 2012;59:1461–1468. doi: 10.1016/j.neuroimage.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Mills KL. Bathula D. Dias TGC. Iyer SP. Fenesy MC. Musser ED. Stevens CA. Thurlow BL. Carpenter SD. Nagel BJ. Nigg JT. Fair DA. Altered cortico-striatal–thalamic connectivity in relation to spatial working memory capacity in children with ADHD. Front Psychiatry. 2012;3:2. doi: 10.3389/fpsyt.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS. Peltier SJ. Wiggins JL. Weng S-J. Carrasco M. Risi S. Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K. Birn RM. Handwerker DA. Jones TB. Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisser U. Boodoo G. Bouchard TJ., Jr. Boykin AW. Brody N. Ceci SJ. Halpern DF. Loehlin JC. Perloff R. Sternberg RJ. Urbina S. Intelligence: knowns and unknowns. Am Psychol. 1996;51:77–101. [Google Scholar]
- Onoda K. Ishihara M. Yamaguchi S. Decreased functional connectivity by aging is associated with cognitive decline. J Cogn Neurosci. 2012;24:2186–2198. doi: 10.1162/jocn_a_00269. [DOI] [PubMed] [Google Scholar]
- Otti A. Guendel H. Läer L. Wohlschlaeger AM. Lane RD. Decety J. Zimmer C. Henningsen P. Noll-Hussong M. I know the pain you feel—how the human brain's default mode predicts our resonance to another's suffering. Neuroscience. 2010;169:143–148. doi: 10.1016/j.neuroscience.2010.04.072. [DOI] [PubMed] [Google Scholar]
- Pagnoni G. Dynamical properties of BOLD activity from the ventral posteromedial cortex associated with meditation and attentional skills. J Neurosci. 2012;32:5242–5249. doi: 10.1523/JNEUROSCI.4135-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi L. Rocca MA. Valsasina P. Panicari L. Mattioli F. Filippi M. Cognitive rehabilitation correlates with the functional connectivity of the anterior cingulate cortex in patients with multiple sclerosis. Brain Imaging Behav. 2012 doi: 10.1007/s11682-012-9160-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Park C. Chang WH. Ohn SH. Kim ST. Bang OY. Pascual-Leone A. Kim Y-H. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011;42:1357–1362. doi: 10.1161/STROKEAHA.110.596155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravatà E. Sestieri C. Mantini D. Briganti C. Colicchio G. Marra C. Colosimo C. Tartaro A. Romani GL. Caulo M. Functional connectivity MR imaging of the language network in patients with drug-resistant epilepsy. AJNR. Am J Neuroradiol. 2011;32:532–540. doi: 10.3174/ajnr.A2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi R. Zhang LJ. Xu Q. Zhong J. Wu S. Zhang Z. Liao W. Ni L. Zhang Z. Chen H. Zhong Y. Jiao Q. Wu X. Fan X. Liu Y. Lu G. Selective impairments of resting-state networks in minimal hepatic encephalopathy. PLoS One. 2012;7:e37400. doi: 10.1371/journal.pone.0037400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C. Liao W. Ding J. Feng Y. Zhu C. Nie X. Zhang W. Chen H. Gong Q. Regional homogeneity changes in social anxiety disorder: a resting-state fMRI study. Psychiatry Res. 2011;194:47–53. doi: 10.1016/j.pscychresns.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Raichle ME. A paradigm shift in functional brain imaging. J Neurosci. 2009;29:12729–12734. doi: 10.1523/JNEUROSCI.4366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Raichle ME. MacLeod AM. Snyder AZ. Powers WJ. Gusnard DA. Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G. Csernansky JG. Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 2011;69:967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann A. Karlsson S. Fischer H. Bäckman L. Caudate dopamine D1 receptor density is associated with individual differences in frontoparietal connectivity during working memory. J Neurosci. 2011;31:14284–14290. doi: 10.1523/JNEUROSCI.3114-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl V. Valet M. Wöller A. Sorg C. Vogel D. Sprenger T. Boecker H. Wohlschläger AM. Tölle TR. Repeated pain induces adaptations of intrinsic brain activity to reflect past and predict future pain. Neuroimage. 2011;57:206–213. doi: 10.1016/j.neuroimage.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Rocca MA. Valsasina P. Absinta M. Riccitelli G. Rodegher ME. Misci P. Rossi P. Falini A. Comi G. Filippi M. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology. 2010;74:1252–1259. doi: 10.1212/WNL.0b013e3181d9ed91. [DOI] [PubMed] [Google Scholar]
- Rosazza C. Minati L. Resting-state brain networks: literature review and clinical applications. Neurol Sci. 2011;32:773–785. doi: 10.1007/s10072-011-0636-y. [DOI] [PubMed] [Google Scholar]
- Rotarska-Jagiela A. Van de Ven V. Oertel-Knöchel V. Uhlhaas PJ. Vogeley K. Linden DEJ. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117:21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Rowe G. Hirsh JB. Anderson AK. Positive affect increases the breadth of attentional selection. Proc Natl Acad Sci U S A. 2007;104:383–388. doi: 10.1073/pnas.0605198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M. Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Ryan JP. Sheu LK. Gianaros PJ. Resting state functional connectivity within the cingulate cortex jointly predicts agreeableness and stressor-evoked cardiovascular reactivity. Neuroimage. 2011;55:363–370. doi: 10.1016/j.neuroimage.2010.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad Z. Gotts SJ. Murphy K. Chen G. Jo HJ. Martin A. Cox R. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Llonch R. Peña-Gómez C. Arenaza-Urquijo EM. Vidal-Piñeiro D. Bargalló N. Junqué C. Bartrés-Faz D. Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex. 2012;48:1187–1196. doi: 10.1016/j.cortex.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Scheid R. Preul C. Gruber O. Wiggins C. Cramon V. Yves D. Diffuse axonal injury associated with chronic traumatic brain injury: evidence from T2*-weighted gradient-echo imaging at 3 T. Am J Neuroradiol. 2003;24:1049–1056. [PMC free article] [PubMed] [Google Scholar]
- Schmitt A. Hasan A. Gruber O. Falkai P. Schizophrenia as a disorder of disconnectivity. Eur Arch Psychiatry Clin Neurosci. 2011;261:150–154. doi: 10.1007/s00406-011-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW. De Rosa E. Anderson AK. Opposing influences of affective state valence on visual cortical encoding. J Neurosci. 2009;29:7199–7207. doi: 10.1523/JNEUROSCI.5387-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW. Selective functional, regional, and neuronal vulnerability in frontotemporal dementia. Curr Opin Neurol. 2008;21:701–707. doi: 10.1097/WCO.0b013e3283168e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW. Menon V. Schatzberg AF. Keller J. Glover GH. Kenna H. Reiss AL. Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon BJ. Raichle ME. Snyder AZ. Fair DA. Mills KL. Zhang D. Bache K. Calhoun VD. Nigg JT. Nagel BJ. Stevens AA. Kiehl KA. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proc Natl Acad Sci U S A. 2011;108:11241–11245. doi: 10.1073/pnas.1108241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ. Beckmann CF. Greenwood R. Kinnunen KM. Bonnelle V. De Boissezon X. Powell JH. Counsell SJ. Patel MC. Leech R. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011;134:2233–2247. doi: 10.1093/brain/awr175. [DOI] [PubMed] [Google Scholar]
- Shehzad Z. Kelly AMC. Reiss PT. Gee DG. Gotimer K. Uddin LQ. Lee SH. Margulies DS. Roy AK. Biswal BB. Petkova E. Castellanos FX. Milham MP. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P. Jagannathan K. Anderson K. Stevens MC. Calhoun VD. Skudlarska BA. Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68:61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fox PT. Miller KL. Glahn DC. Fox PM. Mackay CE. Filippini N. Watkins KE. Toro R. Laird AR. Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M. Zhou Y. Li J. Liu Y. Tian L. Yu C. Jiang T. Brain spontaneous functional connectivity and intelligence. Neuroimage. 2008;41:1168–1176. doi: 10.1016/j.neuroimage.2008.02.036. [DOI] [PubMed] [Google Scholar]
- Stern ER. Fitzgerald KD. Welsh RC. Abelson JL. Taylor SF. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS One. 2012;7:e36356. doi: 10.1371/journal.pone.0036356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens AA. Tappon SC. Garg A. Fair DA. Functional brain network modularity captures inter- and intra-individual variation in working memory capacity. PLoS One. 2012a;7:e30468. doi: 10.1371/journal.pone.0030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC. Lovejoy D. Kim J. Oakes H. Kureshi I. Witt ST. Multiple resting state network functional connectivity abnormalities in mild traumatic brain injury. Brain Imaging Behav. 2012b;6:293–318. doi: 10.1007/s11682-012-9157-4. [DOI] [PubMed] [Google Scholar]
- Stevens WD. Buckner RL. Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cereb Cortex. 2010;20:1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susskind JM. Lee DH. Cusi A. Feiman R. Grabski W. Anderson AK. Expressing fear enhances sensory acquisition. Nat Neurosci. 2008;11:843–850. doi: 10.1038/nn.2138. [DOI] [PubMed] [Google Scholar]
- Tambini A. Ketz N. Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L. Ge Y. Sodickson DK. Miles L. Zhou Y. Reaume J. Grossman RI. Thalamic resting-state functional networks: disruption in patients with mild traumatic brain injury. Radiology. 2011;260:831–840. doi: 10.1148/radiol.11110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME. Hamilton JP. Gotlib IH. Stress-induced activation of the HPA axis predicts connectivity between subgenual cingulate and salience network during rest in adolescents. J Child Psychol Psychiatry. 2011;52:1026–1034. doi: 10.1111/j.1469-7610.2011.02422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L. Ren J. Zang Y. Regional homogeneity of resting state fMRI signals predicts Stop signal task performance. Neuroimage. 2012;60:539–544. doi: 10.1016/j.neuroimage.2011.11.098. [DOI] [PubMed] [Google Scholar]
- Tomasi D. Volkow ND. Functional connectivity hubs in the human brain. Neuroimage. 2011;57:908–917. doi: 10.1016/j.neuroimage.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu P-C. Hsieh J-C. Li C-T. Bai Y-M. Su T-P. Cortico-striatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: A resting fMRI study. Neuroimage. 2012;59:238–247. doi: 10.1016/j.neuroimage.2011.07.086. [DOI] [PubMed] [Google Scholar]
- Vahdat S. Darainy M. Milner TE. Ostry DJ. Functionally specific changes in resting-state sensorimotor networks after motor learning. J Neurosci. 2011;31:16907–16915. doi: 10.1523/JNEUROSCI.2737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel MP. Stam CJ. Kahn RS. Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J Neurosci. 2009;29:7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA. Hedden T. Venkataraman A. Evans KC. Lazar SW. Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer IM. Oei NYL. Spinhoven P. Van Buchem MA. Elzinga BM. Rombouts SARB. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology. 2012;37:1039–1047. doi: 10.1016/j.psyneuen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Venkataraman A. Whitford TJ. Westin C-F. Golland P. Kubicki M. Whole brain resting state functional connectivity abnormalities in schizophrenia. Schizophr Res. 2012;139:7–12. doi: 10.1016/j.schres.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraete E. Van den Heuvel MP. Veldink JH. Blanken N. Mandl RC. Hulshoff Pol HE. Van den Berg LH. Motor network degeneration in amyotrophic lateral sclerosis: a structural and functional connectivity study. PLoS One. 2010;5:e13664. doi: 10.1371/journal.pone.0013664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL. Patel GH. Fox MD. Snyder AZ. Baker JT. Van Essen DC. Zempel JM. Snyder LH. Corbetta M. Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Von dem Hagen EAH. Stoyanova RS. Baron-Cohen S. Calder AJ. Reduced functional connectivity within and between “social” resting state networks in autism spectrum conditions. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss053. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Laviolette P. O'Keefe K. Putcha D. Bakkour A. Van Dijk KRA. Pihlajamäki M. Dickerson BC. Sperling RA. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010a;51:910–917. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Negreira A. Laviolette P. Bakkour A. Sperling RA. Dickerson BC. Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus. 2010b;20:345–351. doi: 10.1002/hipo.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Song M. Jiang T. Zhang Y. Yu C. Regional homogeneity of the resting-state brain activity correlates with individual intelligence. Neurosci Lett. 2011;488:275–278. doi: 10.1016/j.neulet.2010.11.046. [DOI] [PubMed] [Google Scholar]
- Wang Z. Liang P. Jia X. Jin G. Song H. Han Y. Lu J. Li K. The baseline and longitudinal changes of pcc connectivity in mild cognitive impairment: a combined structure and resting-state fMRI study. PLoS One. 2012a;7:e36838. doi: 10.1371/journal.pone.0036838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Yuan Y. Bai F. You J. Li L. Zhang Z. Abnormal default-mode network in angiotensin converting enzyme D allele carriers with remitted geriatric depression. Behav Brain Res. 2012b;230:325–332. doi: 10.1016/j.bbr.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Wegman J. Janzen G. Neural encoding of objects relevant for navigation and resting state correlations with navigational ability. J Cogn Neurosci. 2011;23:3841–3854. doi: 10.1162/jocn_a_00081. [DOI] [PubMed] [Google Scholar]
- Wei T. Liang X. He Y. Zang Y. Han Z. Caramazza A. Bi Y. Predicting conceptual processing capacity from spontaneous neuronal activity of the left middle temporal gyrus. J Neurosci. 2012;32:481–489. doi: 10.1523/JNEUROSCI.1953-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH. Roberts KC. Visscher KM. Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Weng S-J. Wiggins JL. Peltier SJ. Carrasco M. Risi S. Lord C. Monk CS. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S. Thermenos HW. Milanovic S. Tsuang MT. Faraone SV. McCarley RW. Shenton ME. Green AI. Nieto-Castanon A. Laviolette P. Wojcik J. Gabrieli JDE. Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC. Sambataro F. Vasic N. Schmid M. Thomann PA. Bienentreu SD. Wolf ND. Aberrant connectivity of resting-state networks in borderline personality disorder. J Psychiatry Neurosci. 2011;36:402–411. doi: 10.1503/jpn.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. Andreescu C. Butters MA. Tamburo R. Reynolds CF., 3rd Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. 2011a;194:39–46. doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. Wang L. Chen Y. Zhao C. Li K. Chan P. Changes of functional connectivity of the motor network in the resting state in Parkinson's disease. Neurosci Lett. 2009;460:6–10. doi: 10.1016/j.neulet.2009.05.046. [DOI] [PubMed] [Google Scholar]
- Wu X. Li R. Fleisher AS. Reiman EM. Guan X. Zhang Y. Chen K. Yao L. Altered default mode network connectivity in Alzheimer's disease—a resting functional MRI and bayesian network study. Hum Brain Mapp. 2011b;32:1868–1881. doi: 10.1002/hbm.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C. Bai F. Yu H. Shi Y. Yuan Y. Chen G. Li W. Chen G. Zhang Z. Li S-J. Abnormal insula functional network is associated with episodic memory decline in amnestic mild cognitive impairment. Neuroimage. 2012a;63:320–327. doi: 10.1016/j.neuroimage.2012.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C. Goveas J. Wu Z. Li W. Chen G. Franczak M. Antuono PG. Jones JL. Zhang Z. Li S-J. Neural basis of the association between depressive symptoms and memory deficits in nondemented subjects: resting-state fMRI study. Hum Brain Mapp. 2012b;33:1352–1363. doi: 10.1002/hbm.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C. Li S-J. Shao Y. Fu L. Goveas J. Ye E. Li W. Cohen AD. Chen G. Zhang Z. Yang Z. Identification of hyperactive intrinsic amygdala network connectivity associated with impulsivity in abstinent heroin addicts. Behav Brain Res. 2011;216:639–646. doi: 10.1016/j.bbr.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad M. Eichele T. Lundervold AJ. Lundervold A. Subcortical functional connectivity and verbal episodic memory in healthy elderly—a resting state fMRI study. Neuroimage. 2010;52:379–388. doi: 10.1016/j.neuroimage.2010.03.062. [DOI] [PubMed] [Google Scholar]
- Yu Q. Sui J. Rachakonda S. He H. Gruner W. Pearlson G. Kiehl KA. Calhoun VD. Altered topological properties of functional network connectivity in schizophrenia during resting state: a small-world brain network study. PLoS One. 2011;6:e25423. doi: 10.1371/journal.pone.0025423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K. Qin W. Liu J. Guo Q. Dong M. Sun J. Zhang Y. Liu P. Wang W. Wang Y. Li Q. Yang W. Von Deneen KM. Gold MS. Liu Y. Tian J. Altered small-world brain functional networks and duration of heroin use in male abstinent heroin-dependent individuals. Neurosci Lett. 2010;477:37–42. doi: 10.1016/j.neulet.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Zang Y. Jiang T. Lu Y. He Y. Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Zhang D. Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- Zhang D. Snyder AZ. Fox MD. Sansbury MW. Shimony JS. Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol. 2008;100:1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Wang J. Wu Q. Kuang W. Huang X. He Y. Gong Q. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry. 2011;70:334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Lu G. Zhong Y. Tan Q. Yang Z. Liao W. Chen Z. Shi J. Liu Y. Impaired attention network in temporal lobe epilepsy: a resting FMRI study. Neurosci Lett. 2009;458:97–101. doi: 10.1016/j.neulet.2009.04.040. [DOI] [PubMed] [Google Scholar]
- Zhou J. Greicius MD. Gennatas ED. Growdon ME. Jang JY. Rabinovici GD. Kramer JH. Weiner M. Miller BL. Seeley WW. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. 2010a;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. Yu C. Zheng H. Liu Y. Song M. Qin W. Li K. Jiang T. Increased neural resources recruitment in the intrinsic organization in major depression. J Affect Disord. 2010b;121:220–230. doi: 10.1016/j.jad.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Zhu Q. Zhang J. Luo YLL. Dilks DD. Liu J. Resting-state neural activity across face-selective cortical regions is behaviorally relevant. J Neurosci. 2011;31:10323–10330. doi: 10.1523/JNEUROSCI.0873-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X. Wang X. Xiao J. Liao J. Zhong M. Wang W. Yao S. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71:611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.