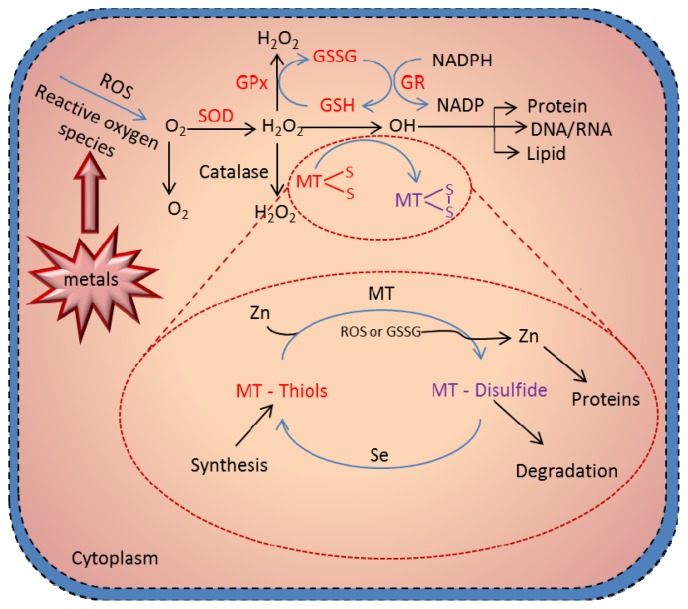
Figure 4.
Metallothionein scavenging of reactive oxygen species. Presence of redox metals, such as Cu and Fe, in a cell can produce reactive oxygen species (ROS), leading to damaging of DNA and cell structures. The cell protects itself using various molecules as scavengers of the radicals. One of the most crucial cell pathways to scavenge the radicals is the glutathione redox complex. However, free –SH moieties of MT can be also involved in the scavenging of ROS in the MT redox cycle. Under physiologic conditions, zinc bound to MT is released through oxidation of the thiolate cluster when the environment becomes oxidized. Formation of MT-disulfide would be subjected to degradation; however, when the oxidized environment became reduced—through, for example, an increase in the glutathione (GSH)/glutathione disulfide (GSSG) ratio—MT disulfide is reduced to MT-thiol. This reduction process is greatly enhanced in the presence of selenium catalyst. In the presence of zinc, MT is quickly reconstituted. This process constitutes the MT redox cycle, which plays a crucial role in the biologic function of MT. Adopted and modified according to [141] and [93].