Figure 3. Identification of inositol 1,4,5-trisphosphate (InsP3)-evoked Ca2+ sparks.
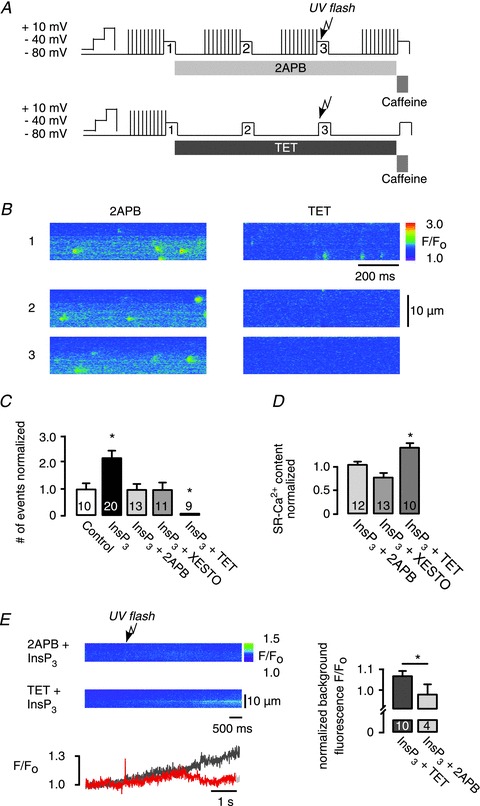
A, experimental protocol, sarcoplasmic reticulum (SR)-Ca2+ loading protocol performed by ICa,L (10 times); atrial myocytes were voltage-clamped under whole-cell conditions, the pipette solution contained 240 μm caged InsP3; line scans are numbered 1–3: (1) control; (2) pharmacological interventions; and (3) pharmacological interventions combined with photolytically released InsP3. Modified protocols were used for the application of 2-aminoethoxydiphenyl borate (2APB) and tetracaine (TET). B, representative data showing line scan images of fluo-3 fluorescence under whole-cell conditions taken at selected time points: (1) control; (2) in the presence of 2APB or TET; (3) pharmacology and photolytic release of InsP3. C, frequency of Ca2+ release events evoked by uncaging of caged InsP3 normalized to control (spontaneous frequency in rest). The increase in Ca2+ spark frequency was inhibited by the application of either 2APB or xestospongin (Xesto; InsP3R inhibitors) and by TET (RyR inhibitor). D, SR-Ca2+ content measured by rapid caffeine (10 mm) application. E, change in background fluo-3 fluorescence F/F0 after InsP3 uncaging, in the presence of TET (dark grey) or 2APB (red). Fluorescence F/F0 was normalized to background fluorescence before InsP3 release (right). Background fluorescence increase after UV-flash photolysis of caged InsP3 in the presence of TET was blocked by 2APB. *P < 0.05 vs. control, N= 2–5; n= 6–13, mean ± SEM.
