Figure 6.
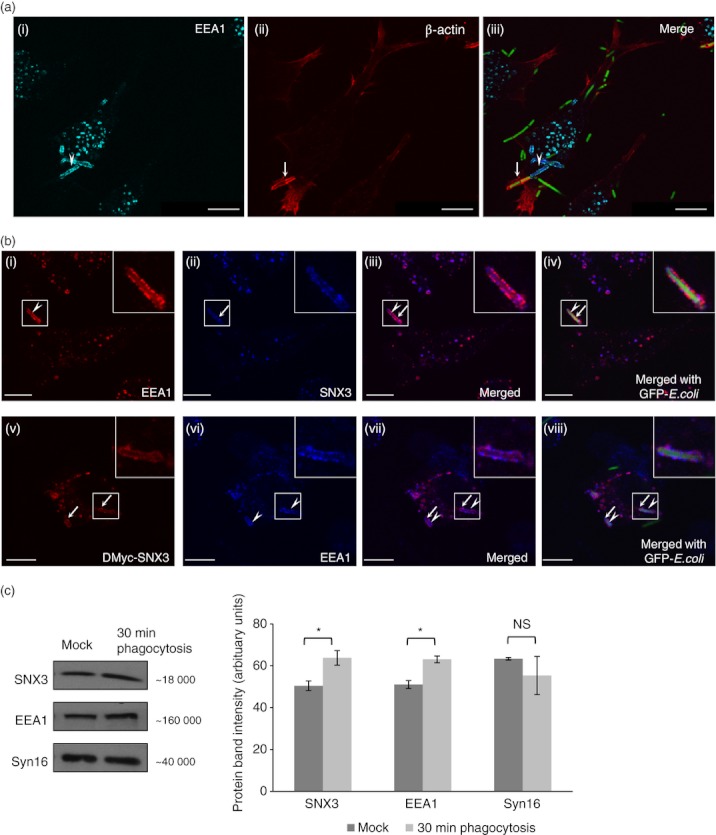
Sorting nexin 3 (SNX3) co-localizes with early endosome antigen-1 (EEA1) on nascent phagosomes. DC2.4 cells were allowed to phagocytose fixed GFP-Escherichia coli at 37° before staining with antibodies against β-actin (monoclonal) and EEA1 (polyclonal, a), or with antibodies against Myc (monoclonal) or SNX3 (polyclonal) and EEA1 (monoclonal or polyclonal, b), followed by respective Cy3-conjugated anti-mouse IgG and AlexaFluor®647-conjugated anti-rabbit IgG. (a) Confocal images showing the co-localization of β-actin (red) and EEA1 (cyan) at the nascent phagosomes. Arrowhead points to the partial enrichment of EEA1 and arrow points to the partial enrichment of β-actin at the nascent phagosome. (b, panel i–iv) Confocal images showing the enrichment of EEA1 (red, arrow head) and SNX3 (blue, arrow) to phagosomes. (b, panel v–viii) Confocal images showing the enrichment of EEA1 (blue, arrow heads) and DMyc-SNX3 (red, arrows) to phagosomes. Inserts are 2·5 times enlargement of the boxed regions. Scale bars: 10 μm. (c) DC2.4 cells were incubated at 37° for 30 min with GFP-E. coli (30-min phagocytosis) or without GFP-E. coli (Mock). After which, phagosome-enriched fractions for both samples were obtained, treated with lysis buffer, and equal amount of proteins were loaded for Western blotting. Comparing ‘30-min phagocytosis’ with ‘Mock’, a significantly greater amount of SNX3 and EEA1 was observed for ‘30-min phagocytosis’, indicating their recruitment to phagosomes. This was not observed in Syn16, which is not known to enrich in phagosomes. A similar trend was observed in two independent experiments. P-value *< 0·05.
