Abstract
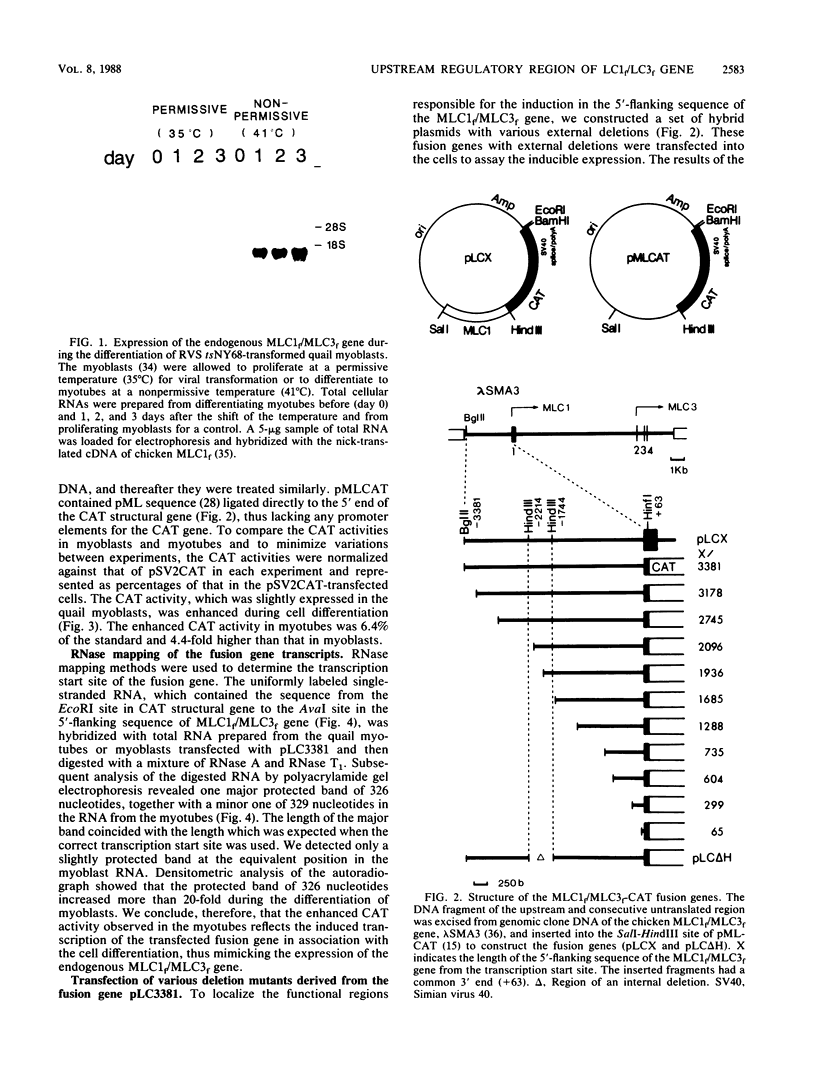
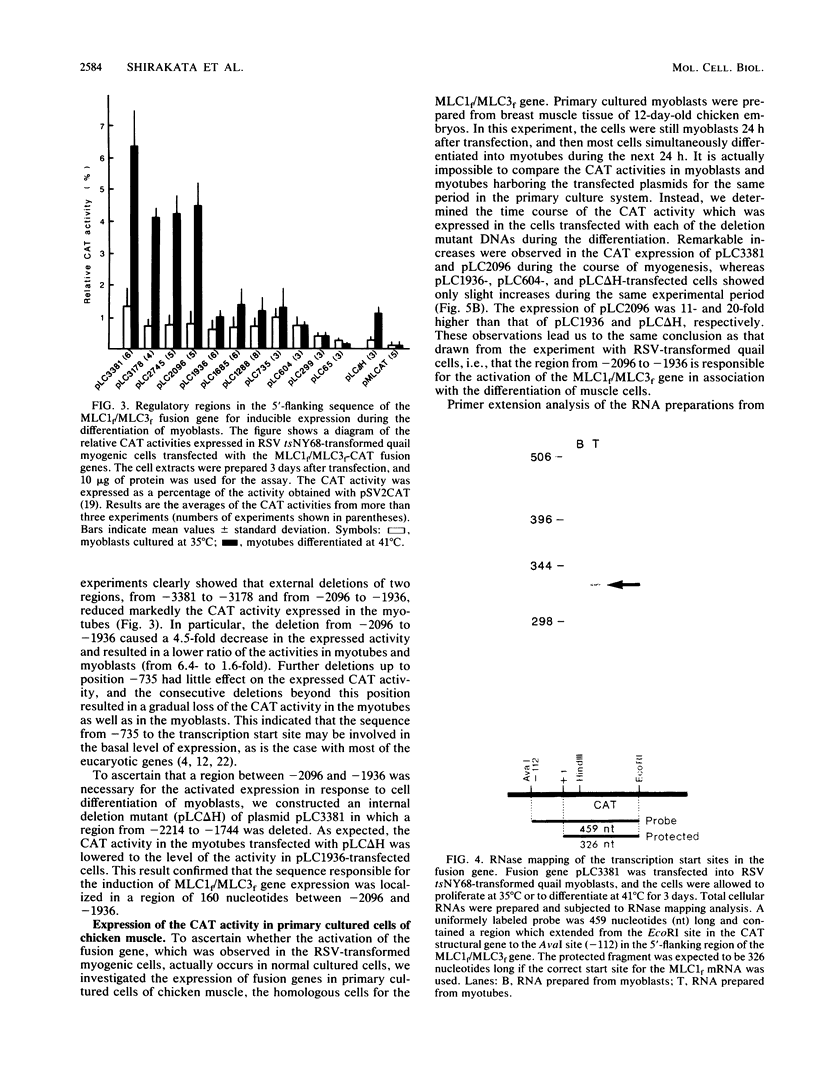
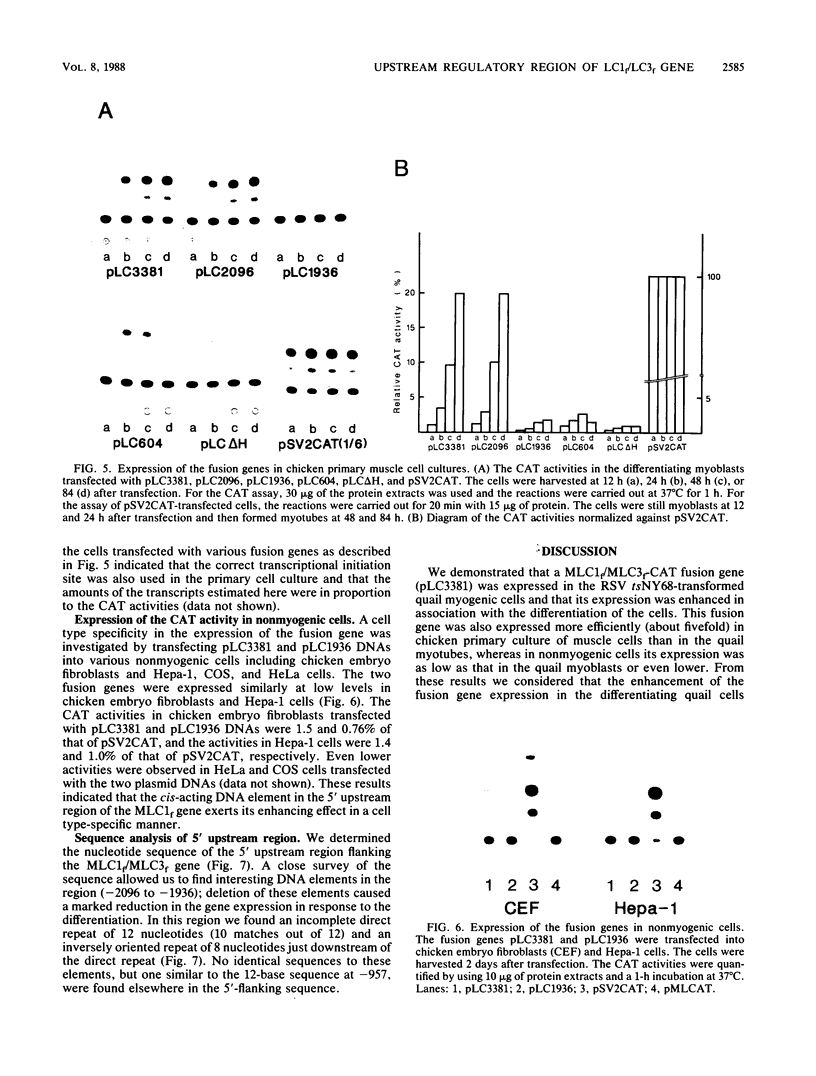
The expression of the fast type of myosin alkali light chain 1 is induced during the differentiation of muscle cells. To study the mechanism of its gene regulation, we joined the sequence of the 5'-flanking and upstream region of the chicken myosin alkali light-chain gene to the structural gene for chloramphenicol acetyltransferase (CAT). The fusion gene was introduced either into quail myoblasts transformed by a temperature-sensitive mutant of Rous sarcoma virus (tsNY68) or into chicken myoblasts, and the transiently expressed CAT activity was assayed after the differentiation of the myoblasts. From the experiments with the external and internal deletion mutants of the fusion gene, the cis-acting regulatory region responsible for the enhanced expression of the CAT activity in response to the cell differentiation was found to be localized at 2 kilobases upstream of the transcription initiation site. This region of 160 nucleotides contained two pairs of short sequences worthy of note, a direct repeat of 12 nucleotides, and an inverted repeat of 8 nucleotides. The nucleotide sequences of the 5'-flanking sequence up to nucleotide -3381 were determined and compared with those of the upstream activating elements of actin genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton P. J., Buckingham M. E. The myosin alkali light chain proteins and their genes. Biochem J. 1985 Oct 15;231(2):249–261. doi: 10.1042/bj2310249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma D. J., Grichnik J. M., Gossett L. M., Schwartz R. J. Delimitation and characterization of cis-acting DNA sequences required for the regulated expression and transcriptional control of the chicken skeletal alpha-actin gene. Mol Cell Biol. 1986 Jul;6(7):2462–2475. doi: 10.1128/mcb.6.7.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M., Pelham H. R. Heat shock regulatory elements function as an inducible enhancer in the Xenopus hsp70 gene and when linked to a heterologous promoter. Cell. 1986 Jun 6;45(5):753–760. doi: 10.1016/0092-8674(86)90789-0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Caravatti M., Minty A., Robert B., Montarras D., Weydert A., Cohen A., Daubas P., Buckingham M. Regulation of muscle gene expression. The accumulation of messenger RNAs coding for muscle-specific proteins during myogenesis in a mouse cell line. J Mol Biol. 1982 Sep;160(1):59–76. doi: 10.1016/0022-2836(82)90131-0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. A 3' enhancer is required for temporal and tissue-specific transcriptional activation of the chicken adult beta-globin gene. Nature. 1986 Oct 23;323(6090):731–734. doi: 10.1038/323731a0. [DOI] [PubMed] [Google Scholar]
- Daubas P., Caput D., Buckingham M., Gros F. A comparison between the synthesis of contractile proteins and the accumulation of their translatable mRNAs during calf myoblast differentiation. Dev Biol. 1981 May;84(1):133–143. doi: 10.1016/0012-1606(81)90377-8. [DOI] [PubMed] [Google Scholar]
- Daubas P., Robert B., Garner I., Buckingham M. A comparison between mammalian and avian fast skeletal muscle alkali myosin light chain genes: regulatory implications. Nucleic Acids Res. 1985 Jul 11;13(13):4623–4643. doi: 10.1093/nar/13.13.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate accumulation of contractile protein mRNAs during myoblast differentiation. Dev Biol. 1979 Mar;69(1):202–216. doi: 10.1016/0012-1606(79)90286-0. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- EPPENBERGER H. M., EPPENBERGER M., RICHTERICH R., AEBI H. THE ONTOGENY OF CREATINE KINASE ISOZYMES. Dev Biol. 1964 Aug;10:1–16. doi: 10.1016/0012-1606(64)90002-8. [DOI] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Sogawa K., Nishi C., Fujii-Kuriyama Y. Regulatory DNA elements localized remotely upstream from the drug-metabolizing cytochrome P-450c gene. Nucleic Acids Res. 1986 Feb 11;14(3):1465–1477. doi: 10.1093/nar/14.3.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Ohno S., Yasumitsu H., Taniguchi T. Delimitation and properties of DNA sequences required for the regulated expression of human interferon-beta gene. Cell. 1985 Jun;41(2):489–496. doi: 10.1016/s0092-8674(85)80022-2. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Changes in protein synthesis during myogenesis in a clonal cell line. Dev Biol. 1979 Nov;73(1):134–152. doi: 10.1016/0012-1606(79)90143-x. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Zinn K., Maniatis T. Human beta-interferon gene expression is regulated by an inducible enhancer element. Cell. 1985 Jun;41(2):509–520. doi: 10.1016/s0092-8674(85)80024-6. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Spacer DNA sequences upstream of the T-A-T-A-A-A-T-A sequence are essential for promotion of H2A histone gene transcription in vivo. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7102–7106. doi: 10.1073/pnas.77.12.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes J. B., Chamberlain J. S., Buskin J. N., Johnson J. E., Hauschka S. D. Transcriptional regulation of the muscle creatine kinase gene and regulated expression in transfected mouse myoblasts. Mol Cell Biol. 1986 Aug;6(8):2855–2864. doi: 10.1128/mcb.6.8.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny S. F., Emerson C. P., Jr Complex regulation of the muscle-specific contractile protein (troponin I) gene. Mol Cell Biol. 1987 Sep;7(9):3065–3075. doi: 10.1128/mcb.7.9.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhara S., Matsuo F., Futamura S., Fujita A., Shinohara T., Takagi T., Sakaki Y. GENAS: a database system for nucleic acid sequence analysis. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):89–99. doi: 10.1093/nar/12.1part1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebherz H. G. Ontogeny and regulation of fructose diphosphate aldolase isoenzymes in "red" and "white" skeletal muscles of the chick. J Biol Chem. 1975 Aug 10;250(15):5976–5981. [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Melloul D., Aloni B., Calvo J., Yaffe D., Nudel U. Developmentally regulated expression of chimeric genes containing muscle actin DNA sequences in transfected myogenic cells. EMBO J. 1984 May;3(5):983–990. doi: 10.1002/j.1460-2075.1984.tb01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T., Kedes L. Duplicated CArG box domains have positive and mutually dependent regulatory roles in expression of the human alpha-cardiac actin gene. Mol Cell Biol. 1987 Aug;7(8):2803–2813. doi: 10.1128/mcb.7.8.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarras D., Fiszman M. Y. A new muscle phenotype is expressed by subcultured quail myoblasts isolated from future fast and slow muscles. J Biol Chem. 1983 Mar 25;258(6):3883–3888. [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Molecular cloning and nucleotide sequences of the complementary DNAs to chicken skeletal muscle myosin two alkali light chain mRNAs. Nucleic Acids Res. 1982 Oct 11;10(19):6099–6110. doi: 10.1093/nar/10.19.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudel U., Greenberg D., Ordahl C. P., Saxel O., Neuman S., Yaffe D. Developmentally regulated expression of a chicken muscle-specific gene in stably transfected rat myogenic cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3106–3109. doi: 10.1073/pnas.82.10.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy M., Strehler E. E., Garfinkel L. I., Gubits R. M., Ruiz-Opazo N., Nadal-Ginard B. Fast skeletal muscle myosin light chains 1 and 3 are produced from a single gene by a combined process of differential RNA transcription and splicing. J Biol Chem. 1984 Nov 10;259(21):13595–13604. [PubMed] [Google Scholar]
- Pfeifer K., Prezant T., Guarente L. Yeast HAP1 activator binds to two upstream activation sites of different sequence. Cell. 1987 Apr 10;49(1):19–27. doi: 10.1016/0092-8674(87)90751-3. [DOI] [PubMed] [Google Scholar]
- Robert B., Daubas P., Akimenko M. A., Cohen A., Garner I., Guenet J. L., Buckingham M. A single locus in the mouse encodes both myosin light chains 1 and 3, a second locus corresponds to a related pseudogene. Cell. 1984 Nov;39(1):129–140. doi: 10.1016/0092-8674(84)90198-3. [DOI] [PubMed] [Google Scholar]
- Robert B., Weydert A., Caravatti M., Minty A., Cohen A., Daubas P., Gros F., Buckingham M. cDNA recombinant plasmid complementary to mRNAs for light chains 1 and 3 of mouse skeletal muscle myosin. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2437–2441. doi: 10.1073/pnas.79.8.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salviati G., Betto R., Danieli Betto D. Polymorphism of myofibrillar proteins of rabbit skeletal-muscle fibres. An electrophoretic study of single fibres. Biochem J. 1982 Nov 1;207(2):261–272. doi: 10.1042/bj2070261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfling E., Lübbe A., Dorsch-Häsler K., Schaffner W. Metal-dependent SV40 viruses containing inducible enhancers from the upstream region of metallothionein genes. EMBO J. 1985 Dec 30;4(13B):3851–3859. doi: 10.1002/j.1460-2075.1985.tb04157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani M., Zevin-Sonkin D., Saxel O., Carmon Y., Katcoff D., Nudel U., Yaffe D. The correlation between the synthesis of skeletal muscle actin, myosin heavy chain, and myosin light chain and the accumulation of corresponding mRNA sequences during myogenesis. Dev Biol. 1981 Sep;86(2):483–492. doi: 10.1016/0012-1606(81)90206-2. [DOI] [PubMed] [Google Scholar]
- Sogawa K., Fujisawa-Sehara A., Yamane M., Fujii-Kuriyama Y. Location of regulatory elements responsible for drug induction in the rat cytochrome P-450c gene. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8044–8048. doi: 10.1073/pnas.83.21.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler E. E., Periasamy M., Strehler-Page M. A., Nadal-Ginard B. Myosin light-chain 1 and 3 gene has two structurally distinct and differentially regulated promoters evolving at different rates. Mol Cell Biol. 1985 Nov;5(11):3168–3182. doi: 10.1128/mcb.5.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Yablonka Z., Yaffe D. Synthesis of myosin light chains and accumulation of translatable mRNA coding for light chain-like polypeptides in differentiating muscle cultures. Differentiation. 1977 Oct 13;8(3):133–143. doi: 10.1111/j.1432-0436.1977.tb00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarraga A. M., Danishefsky K., Deshpande A., Nicholson D., Mendola C., Siddiqui M. A. Characterization of 5'-flanking region of heart myosin light chain 2A gene. Structural and functional evidence for promoter activity. J Biol Chem. 1986 Oct 15;261(29):13852–13860. [PubMed] [Google Scholar]