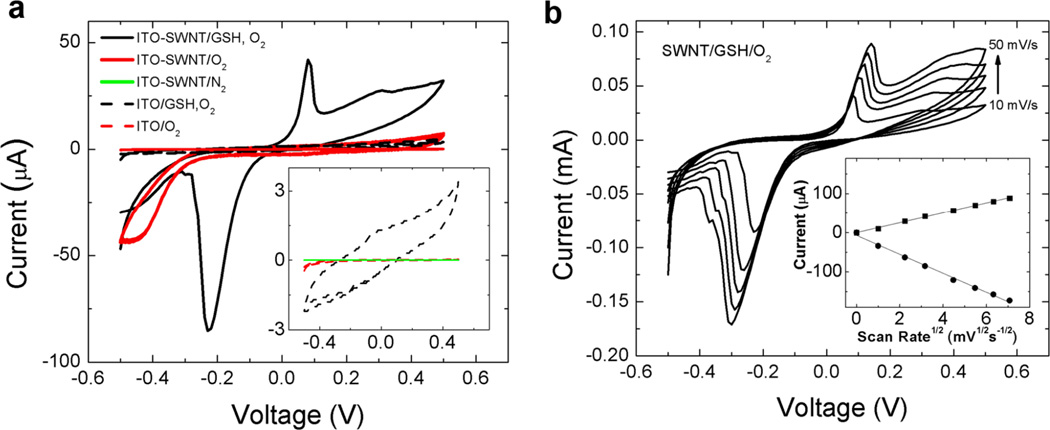
Figure 7.
(a) Cyclic voltammogram (CV) of glutathione/glutathione disulfide (GSH/GSSG) redox couple at an ITO electrode coated with single-walled carbon nanotubes (SWNT). Quasi-reversible electrochemistry is indicated by the spread in the anodic and cathodic peaks centered at 0.1V and −0.23V, respectively. CVs of control experiments are included for comparison. CV of ITO-SWNT with and without O2 present reveals the onset potential for O2 reduction at −0.24 V. The inset expands the CVs of the three control experiments exhibiting low currents. A lack of faradaic current in these control experiments indicates both SWNT and O2 must be present to observe GSH/GSSG electrochemistry. The onset potentials for GSSG reduction and GSH oxidation are −0.05 V and +0.05 V, respectively. Note that the region associated with O2 reduction in this CV is depleted relative to the CV without GSH at identical concentrations of O2. Reference electrode: Ag/AgCl; scan rate 10 mV/s, counter electrode carbon felt. (b) Electrochemical behavior of the SWNT-coated ITO/O2/GSH system: effect of scan rate. Current is reduced at low scan rate indicating local reactant depletion. The linear dependence of peak current on the square root of scan rate indicates the rate-limiting electrochemical process involves diffusible GSH/GSSG. All data are for purified SWNTs (329 m2/g).