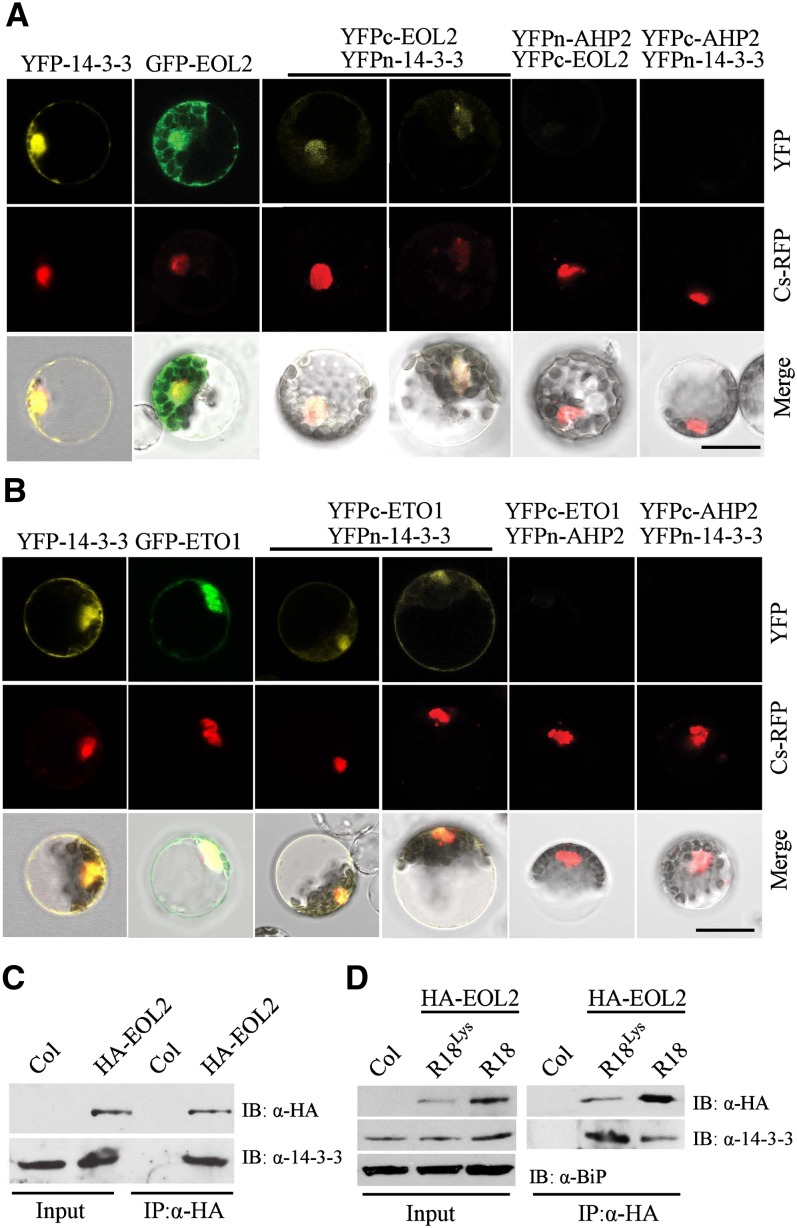
Figure 3.
14-3-3 Interacts with ETO1/EOLs in Vivo.
(A) and (B) BiFC analysis of 14-3-3ω interacting with ETO1 (A) or EOL2 (B). The left-most panel shows a full-length YFP fused to 14-3-3ω. The second panel shows a full-length GFP fused to EOL2 (A) or ETO1 (B). The remaining panels show the result of cotransformation with the indicated BiFC plasmids. A Cs-RFP plasmid was used as a marker for the nucleus and transformation control. The top row shows the YFP or GFP signal, the second row the RFP signal, and the lowest row a merge of the GFP/YFP, RFP, and differential interference contrast images. Bars = 20 µm.
(C) Coimmunoprecipitation of EOL2 and 14-3-3 proteins. Protein extracts from Arabidopsis seedlings expressing HA-EOL2 were immunoprecipitated with an anti-HA antibody, and the immunoprecipitated proteins were analyzed by immunoblotting using an anti-HA or an anti-14-3-3 antibody. Col, Columbia.
(D) R18 peptide disrupts the interaction between EOL2 and 14-3-3 proteins. Arabidopsis seedlings expressing HA-EOL2 were treated with 20 µg/mL R18 or R18Lys peptide, total protein extracts were immunoprecipitated (IP) with an anti-HA antibody, and the immunoprecipitated proteins were analyzed by immunoblotting (IB) using an anti-HA or anti-14-3-3 antibody.