Figure 4.
14-3-3 Destabilizes EOL2 Protein Stability.
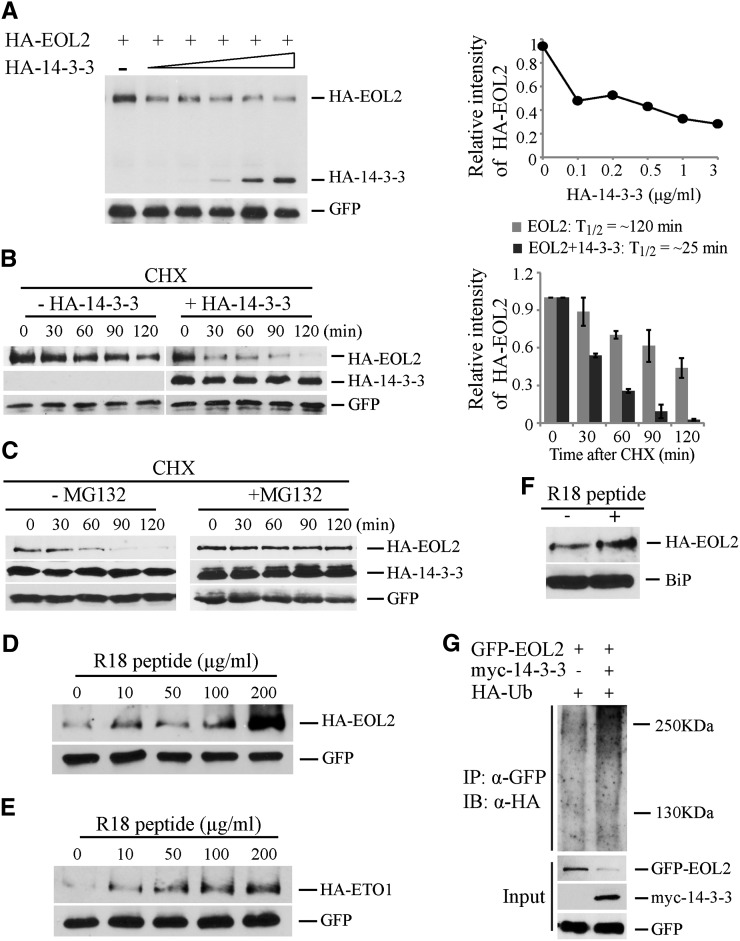
(A) 14-3-3 destabilizes EOL2 protein. Protein extracts from protoplasts transformed with plasmid expressing HA-EOL2, and increasing amounts of plasmid expressing HA-14-3-3ω were analyzed by immunoblotting with an anti-HA or anti-GFP antibody, which was used as a transformation control. The right shows a quantification of the immunoblots. The HA-EOL2 signal was normalized to the GFP signal, and these values expressed relative to the no HA-14-3-3ω control, which was set to 1.
(B) 14-3-3 decreases the half-life of EOL2 protein. Protoplasts were transformed with a plasmid expressing HA-EOL2 with or without a plasmid expressing 14-3-3ω. At time 0, cycloheximide was added (250 µM Cf), and total proteins were extracted at the indicated times and then analyzed by immunoblotting using the indicated antibodies. Right: The HA-EOL2 signals were normalized to the matching GFP signals and these values expressed relative to the time 0 value, which was set to 1. A quantification of the protein levels from three independent biological replicates using ImageJ software is shown on right. Values are presented as the mean ± se.
(C) 14-3-3–mediated destabilization of EOL2 protein is ubiquitin/proteasome dependent. Protoplasts expressing HA-EOL2 and HA-14-3-3ω were incubated with or without MG132 for the indicated times, and total proteins were extracted at the indicated times and then analyzed by immunoblotting using the indicated antibodies.
(D) and (E) R18 peptide promotes accumulation of EOL2 (D) and ETO1 (E) proteins. The indicated concentrations of R18 peptide were added to the protoplasts transformed with plasmids expressing either HA-EOL2 or HA-ETO1. The blots were probed with anti-HA and anti-GFP antibodies.
(F) R18 peptide promotes accumulation of EOL2 protein in planta. Arabidopsis seedlings expressing HA-EOL2 were treated with 20 µg/mL R18 peptide or a mock control. Total protein was extracted and analyzed by immunoblotting using an anti-HA antibody or anti-BiP antibody as a loading control.
(G) 14-3-3 promotes the ubiquitination of EOL2 protein. Protoplasts expressing GFP-EOL2 and HA-ubiquitin in the presence or absence of a myc-14-3-3ω expression plasmids as indicated were incubated with 50 µM MG132 for 6 h. The protein extracts were immunoprecipitated (IP) with an anti-GFP antibody and then analyzed by immunoblotting (IB) using an anti-HA or anti-GFP antibody. The GFP signal is used as a transformation control.