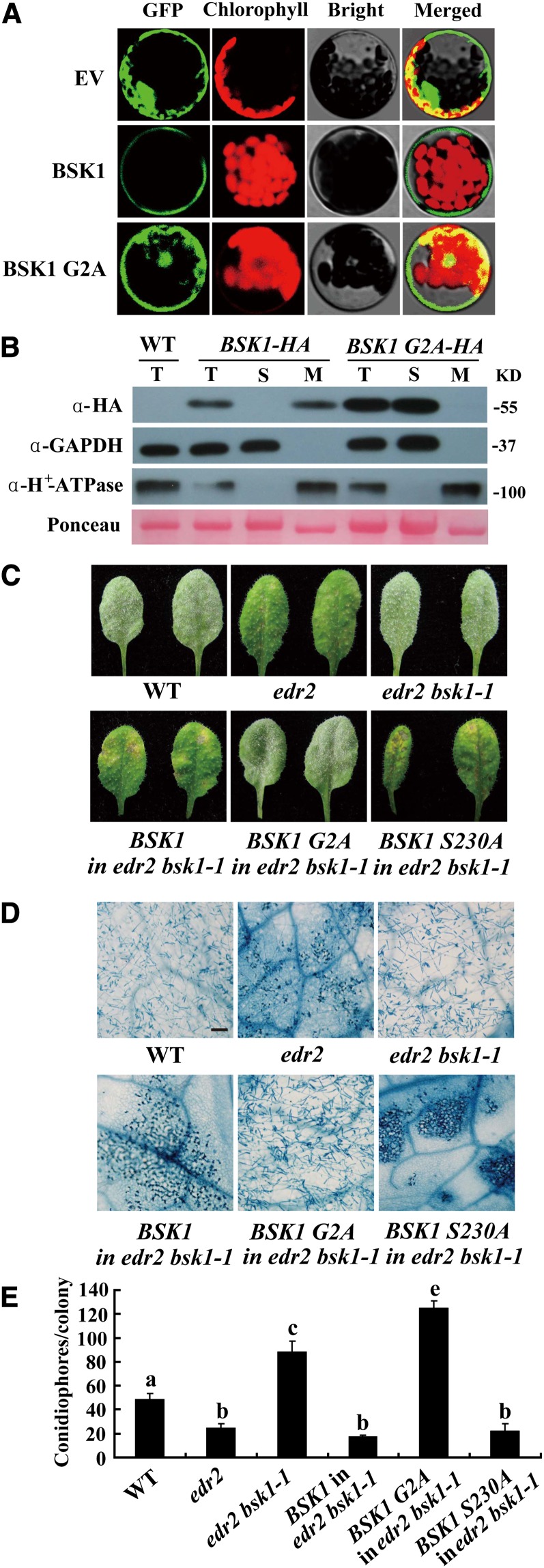
Figure 4.
BSK1-GFP Localizes to the Plasma Membrane, and Disruption of the Predicted Myristoylation Site Compromises BSK1 Function.
(A) BSK1-GFP was transformed into Arabidopsis protoplasts, and the GFP signal was detected by confocal microscopy. BSK1-GFP localizes to cell periphery, and disruption of the myristoylation site of BSK1 (G2A) compromised BSK1 cell periphery localization. EV, empty vector.
(B) Subcellular fractionation and immunoblot assays. Total protein was extracted from 4-week-old plants. Total (T), soluble (S), and membrane (M) fractions of protein from BSK1-HA or BSK1 G2A-HA transgenic plants were loaded on SDS-PAGE gels and subjected to immunoblotting with anti-HA antibody. GAPDH and H+-ATPase were used as soluble marker or plasma membrane marker, respectively. Molecular masses of protein markers are shown on the right. Ponceau S staining of ribulose-1,5-bis-phosphate carboxylase/oxygenase is shown as a loading control. The experiments were repeated three times with similar results. WT, the wild type.
(C) to (E) The BSK1 G2A-HA clone was unable to restore edr2 bsk1-1 to the edr2 phenotype. Four-week-old plants were infected with G. cichoracearum.
(C) Representative leaves were removed and photographed at 8 DAI. Leaves of two independent transgenic lines for each construct are shown. More than 10 independent transgenic lines expressing the fusion proteins of the correct sizes were examined for each construct. All the transgenic lines examined showed consistent phenotypes.
(D) The infected leaves at 8 DAI were stained with trypan blue. Bar = 100 μm.
(E) The number of conidiophores per colony was counted at 5 DAI. Bars represent mean and sd (n > 30). Lowercase letters represent statistically significant differences (P < 0.01, one-way ANOVA). The experiments were repeated three times with similar results.