Figure 1.
RNA-Mediated Silencing of the AOC5 Gene Induced by Expression of AOC5 IR Transgenes in C. reinhardtii Transformants.
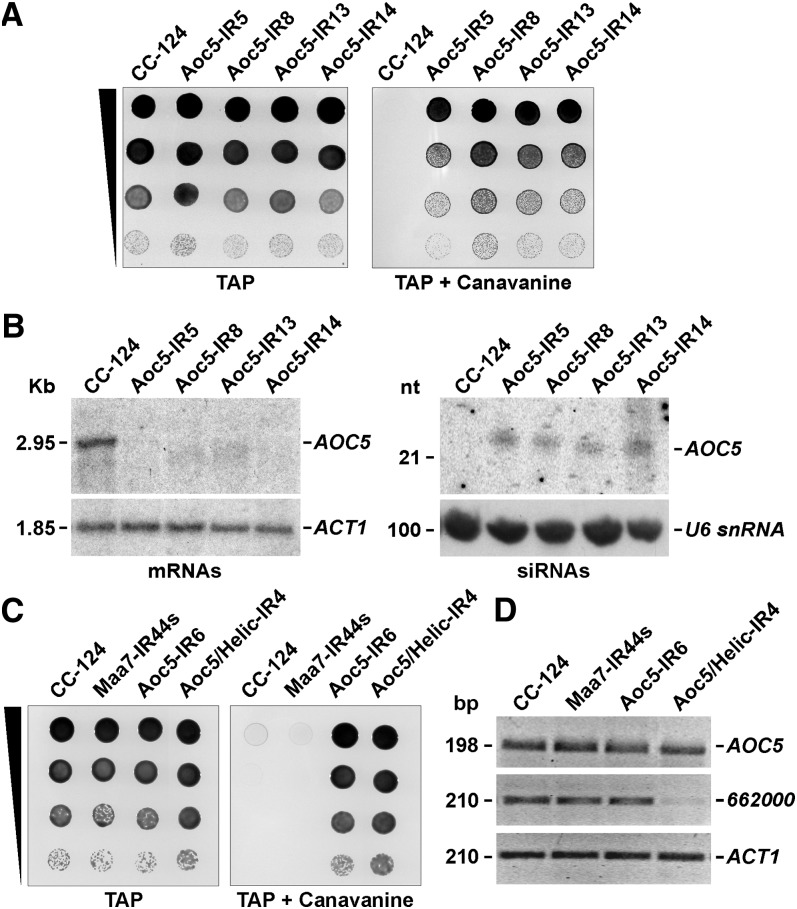
(A) Growth and survival of Aoc5-IR transformants on TAP medium without (left) or with (right) 400 μg/mL l-canavanine. CC-124, untransformed wild-type strain.
(B) RNA gel blot analyses of mRNAs and siRNAs in the Aoc5-IR transgenic strains. The left panels correspond to agarose gel–separated total RNA samples sequentially hybridized with 32P-labeled PCR products corresponding to the AOC5 3′ UTR (top panel), to evaluate the degree of mRNA reduction, or the coding sequence of Actin (ACT1) (bottom panel) as a control for equivalent loading of the lanes. The right panels correspond to total RNA samples separated in a 15% denaturing polyacrylamide gel and probed sequentially with the AOC5 3′ UTR sequence (top panel), to detect siRNAs, or the U6 small nuclear RNA sequence (bottom panel), to assess the amount of sample loaded per lane. nt, nucleotides.
(C) Growth and survival of the indicated strains on TAP medium alone or containing 400 μg/mL l-canavanine. Maa7-IR44s, strain containing an IR transgene targeting the 3′ UTR of the MAA7 gene (encoding TSβ). Aoc5/Helic-IR4, strain containing a tandem IR transgene targeting both AOC5 and Cre16.g662000 (encoding a putative RNA helicase).
(D) RT-PCR analyses on total RNA samples from the indicated strains. The panels show reverse images of agarose gel–fractionated RT-PCR products corresponding to AOC5 or Cre16.g662000. Amplification of the mRNA corresponding to ACT1 was used as a control for equal amounts of input RNA and for the efficiency of the RT-PCRs (bottom panel). Reactions using RNA not treated with reverse transcriptase as the template were employed as a negative control (data not shown).