Fig. 1. αB-crystallin R120G has decreased and αB-crystallin M68A increased chaperone activity levels relative to wt αB-crystallin.
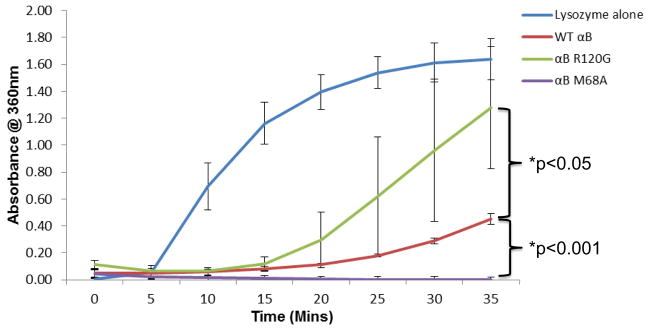
Chaperone activities of purified recombinant wt αB-crystallin, αB-crystallin R120G, and αB-crystallin M68A using lysozyme as a target substrate. Lysozyme was incubated in the presence or absence of wt αB-crystallin or αB-crystallin mutants R120G and M68A in the presence of absence of 20 mM DTT and protein aggregation monitored by measuring absorbance (turbidity) at 360nm. Graph shows mean values of n=3 ± SD. p values were calculated using two sample Student t-test assuming equal variance. Significant differences between wt and mutants are shown wt - R120G, p<0.05, wt - M68A, p<0.001.
