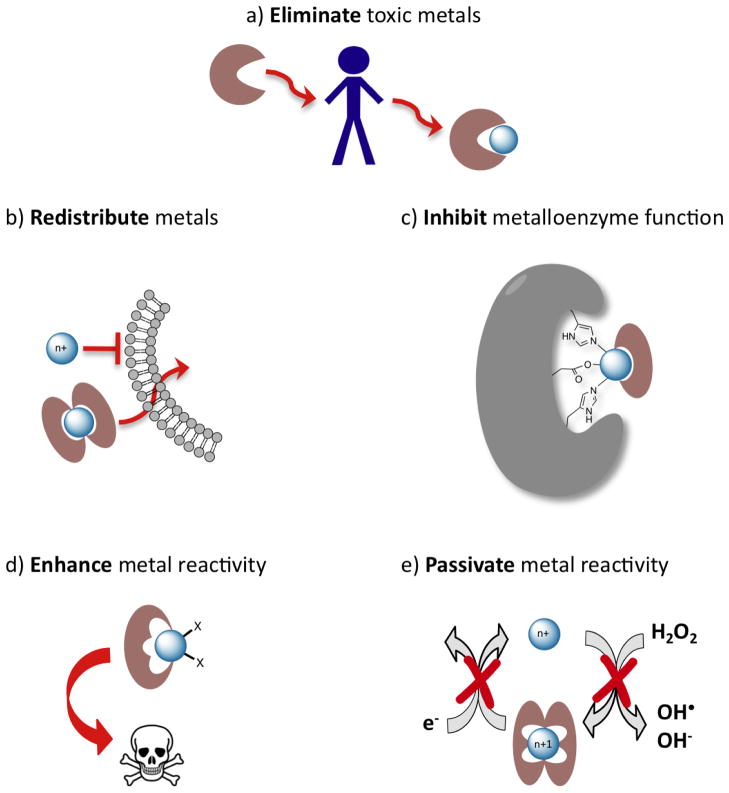
Figure 1.
Not all chelating agents are the same. (a) The traditional notion of chelation therapy as reducing the total body burden of a heavy metal. Other approaches that use principles of metal chelation include (b) redistributing a metal across biological membranes via formation of neutral, lipophilic complexes; (c) inactivating an enzyme active site by taking advantage of metal chelation to form protein-metal-chelator ternary complexes; (d) enhancing the reactivity of a metal by forming a chelate complex, for example that promotes redox cycling and generation of reactive oxygen species or other cytotoxic products; (e) inactivating the reactivity of a metal, for example by forming a chelate complex that prevents Fenton chemistry where redox cycling catalyzes formation of reactive oxygen species. Each example shows a generic metal, as these approaches can in principle apply across the periodic table.