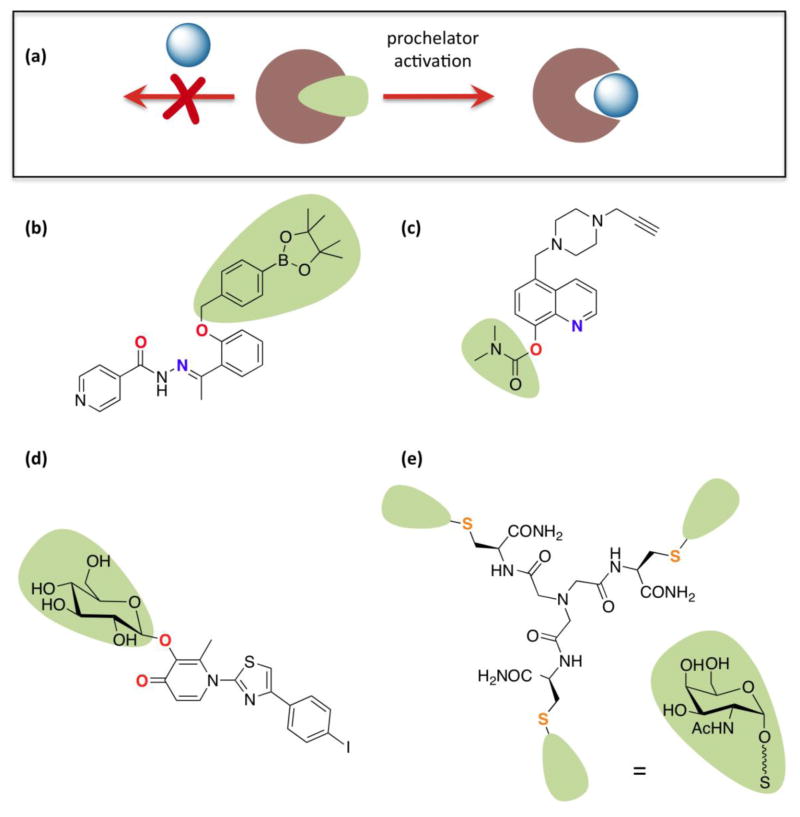
Figure 4.
(a) General prochelator strategy, where a masking group blocks metal binding until activation releases the chelating unit. For specific prochelator examples, the atoms ultimately involved in metal chelation are in bold and color-coded; (b) BHAPI, which can be activated by H2O2;[33] (c) HLA20A, which is activated by acetylcholinesterase and has multifunctional neurorescueing properties;[35] (d) a multifunctional, amyloid targeting prochelator activated by glucosidase enzymes;[37] (e) a tripodal glycoconjugate that targets hepatocytes and is reductively activated intracellularly to release a Cu(I) chelator (squiggly line represents ethylene glycol linker).[38]