Table 1.
Initial phenylthiophene SAR vs. aPKCζa
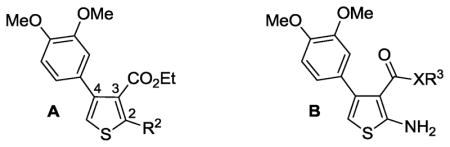
| |||
|---|---|---|---|
| No. | Str. | R2 or XR3 | Inh (%)a |
| 123 | A | NH2 | 100 |
| 2 | A | H | 3 |
| 3 | A | NHMe | 32 |
| 4 | A | NMe2 | 60 |
| 5 | A | NHCONH2 | 14 |
| 6 | B | O-i-Pr | 100 |
| 7 | B |
|
57 |
| 8 | B |
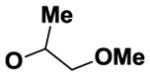
|
49 |
| 9 | B | OPh | 48 |
| 10 | B | OCH2Ph | 100 |
| 11 | B | OCH2Ph-2-Me | 64 |
| 12 | B | OCH2Ph-3-Me | 67 |
| 13 | B | OCH2Ph-4-Me | 50 |
| 14 | B | OCH2Ph-3-OMe | 100 |
| 15 | B | OCH2Ph-2-F | 100 |
| 16 | B | OCH2Ph-4-F | 2 |
| 17 | B | OCH2Ph-3-CN | 70 |
| 18 | B | OCH2Ph-4-CN | 10 |
| 19 | B | OCH2-4-pyridyl | 53 |
| 20 | B | NHEt | 50 |
| 21 | B |
|
55 |
| 22 | B | NHCH2Ph | 43 |
| 23 | B | NHCH2-4-pyridyl | 3 |
| 24a24 | B | COXR3 = CN | 87 |
| 24b | B | COXR3 = CO2H | 65 |
Inhibition determined from ADP Quest assay with 30 μM of inhibitor using aPKCζ (500 ng/ml). Values are the mean of at least n≥5 and were repeated in independent experiments. Standard error of the mean <20%. The experimental conditions are reported in the Supplementary Material.
Str. = chemical structure.
