Table 2.
Dehydrogenation of Substituted Cyclohexenones,[a]
 | ||||
|---|---|---|---|---|
| Entry | Cyclohexenone | Phenol | Time [h] |
Yield [%][b] |
| 1 | 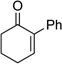 |
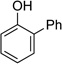 |
10 | 90[c] |
| 2 | 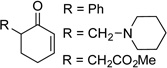 |
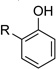 |
10 | 96[c] |
| 3 | 36 | 46 | ||
| 4 | 6.5 | 63 | ||
| 5 | 10 | 87 | ||
| 6 | 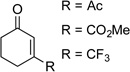 |
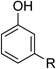 |
24 | 77 |
| 7 | 24 | 73 | ||
| 8 | 12 | 68 | ||
| 9 |  |
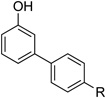 |
10 | 89 |
| 10 | 10 | 52[c] | ||
| 11 | 10 | 83 | ||
Reactions were performed on 1.0 mmol scale in DMSO/H2O (v/v 0.2/0.05 mL).
Isolated yield.
PdII/L5/AMS = 5%/5%/15%.
