Table 3.
Optimization of Conditions for the Oxidative Heck and one-pot Oxidative Heck/Dehydrogenation Reactions.[a]
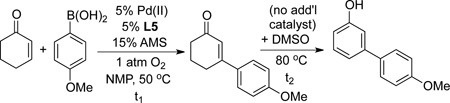 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Oxidative Heck | Oxidative Heck/ Dehydrogenation |
|||||
| Solvent | Conc. [M][b] |
t1 [h] |
Yield [%][c] |
Solvent | t2 [h] |
Yield [%][d] |
|
| 1 | DMSO | 1.3 | 8 | 65 | - | 24 | 64 |
| 2 | DMF | 1.3 | 3 | 95 | - | 24 | 25[e] |
| 3 | NMP | 1.3 | 3 | 90 | - | - | - |
| 4 | Dioxane | 1.3 | 3 | 93 | - | - | - |
| 5 | CH3CN | 1.3 | 3 | 73 | - | - | - |
| 6 | DMF | 2.5 | 3 | 96 | - | - | - |
| 7 | DMF | 5.0 | 3 | 80 | - | - | - |
| 8 | DMF | 2.5 | 3 | - | DMSO | 24 | 54 |
| 9 | NMP | 2.5 | 3 | - | DMSO | 24 | 72 |
| 10 | NMP | 3.1 | 3 | - | DMSO | 24 | 82 |
| 11[f] | NMP | 3.1 | 3 | - | DMSO | 32 | 82 |
| 12[f,g] | NMP | 3.1 | 4 | 95 | DMSO | 32 | 84 |
Reaction conditions: Heck reactions [cyclohexenone (0.75 mmol), boronic acid (0.25 mmol), Pd(II) = [Pd(CH3CN)4](BF4)2, 50 °C, 3–8 h]; Dehydrogenation: temp. increased to 80 °C, 24–32 h (for entries 8–15, 0.2 mL DMSO added).
Conc. of boronic acid.
GC yields.
1H NMR yields.
65% of Heck product recovered.
10% AMS used.
0.5 mmol cyclohexenone.
